Hydrogen through Sunlight
Researchers Develop Sustainable Catalyst System for Hydrogen Production Using Light Energy
For hydrogen to be sustainably produced using sunlight, it's not just an efficient catalyst system that's needed – it must also be economical, readily available, and resource-efficient. A team led by chemist Prof. Dr Kalina Peneva from the Institute of Organic Chemistry and Macromolecular Chemistry at the University of Jena has made a step in this direction. In their research, the group developed dyes that are metal-free, simple to produce, and can transfer absorbed light energy to a catalyst to produce hydrogen.

Prof. Dr Kalina Peneva and PhD student Konrad Hotzel are investigating functional dyes.
Anne Günther/Uni Jena
Innovative Dye Molecules
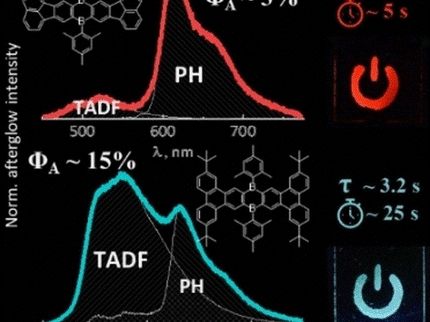
“More precisely, these dye molecules, also called photosensitizers, absorb light and convert its energy into a long-lived excited state within the molecule,” explains Peneva. She compares this process to fluorescence, as seen in objects or garments that glow brightly under black light. “However, our dyes don't release the energy as light,” she qualifies. “Instead, we use this stored energy by transferring it to a specifically adapted catalyst. This then generates hydrogen from water.”
Cost-effective and Environmentally Friendly Catalysts
Catalyst systems that generate hydrogen with sunlight have been developed and researched for many years, including in the Collaborative Research Center “CataLight” funded by the German Research Foundation, which Kalina Peneva's project is also part of. “What's special about our dye molecules, however, is that they work without the addition of noble metals. Often these dyes involve ruthenium or osmium – metals that are rare and expensive. And the catalyst we use, instead of expensive noble metals like platinum or palladium, utilises much more affordable cobalt,” explains Peneva.
Efficiency through Simplified Synthesis
But the production of these sustainable dyes is also promising: “What we have developed is unique because it offers a simple and efficient method of synthesising these molecules, which greatly enhances the scalability of the process,” adds the chemist. “We form the dye through a simple so-called condensation reaction. And as the dye precipitates as a solid, we can easily separate it from the reaction mixture through filtration,” she adds. “Therefore, unlike many other synthesis approaches, there's no need for expensive and complicated purification steps for the desired product.”
Interdisciplinary Collaboration
The research team collaborated closely within the University of Jena with the group of Benjamin Dietzek-Ivanšić from the Institute of Physical Chemistry, systematically examining different variants of the dye to find the optimal configuration for light-driven catalysis. "We not only developed the dyes but also studied their interaction with the catalyst in detail," explains Prof. Peneva.
The results published by the team in the journal “Journal of Materials Chemistry A” are promising: “Although we haven't achieved the highest efficiency, the results are very good and, above all, show great potential for practical applications,” she highlights. “Specifically, we measure this using the turnover number, TON for short; an important metric for the performance of catalysts. And it's at a good value of around 4,000,” she adds.
She and her team are already considering initiating research transfer with potential industrial partners. “Whether we attempt to produce this catalyst system on an industrial scale at this point, I cannot say,” limits Kalina Peneva. “But it's certainly a consideration we are making.”
Original publication
Original publication
Gergely Knorr, Konrad Hotzel, Avinash Chettri, Artem Skabeev, Maria Wächtler, Benjamin Dietzek-Ivanšić, Kalina Peneva; "Unlocking the potential of ketocoumarins: efficient photosensitizers for sustainable light driven hydrogen evolution"; Journal of Materials Chemistry A, Volume 11, 2023
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
A sea of spinning electrons

SilcoTek Corporation - Bellefonte, USA
New device can measure toxic lead within minutes - Researchers create portable lab-on-a-chip that could detect many contaminants
Dow Corning announces personnel updates to its global pressure sensitive industry team