Synergistic roles of platinum nanoparticles and sodium ions within beta zeolites in n-alkylation of amines with aromatic alcohols
N-alkylamines synthesis through N-alkylation of amines with alcohols catalyzed by Beta zeolites encapsulated Pt nanoparticles
Organic N-alkyl amines are important chemical products and intermediate with wide applications in the fields of daily chemicals, pesticides, pharmaceuticals, surfactants, and life sciences. The C–N bonds formed by alkylating reagents (halocarbons, metal halides, etc.) to produce amines usually suffered from low atomic efficiency, poor product selectivity, and chemical contaminants. Sustainable development motivates the exploration of more efficient and eco-friendly routes for amine formation, among which, n-alkylation of amines and alcohols through hydrogen transfer is a green way to synthesize N-alkylamines, but the design and preparation of highly active and stable heterogeneous catalysts is still a challenge. Compared with the traditional impregnation method, the metal encapsulated in zeolite is beneficial to provide a unique constraint effect and produces a synergistic effect with the acid site, favoring improved catalytic activity and stability. For the N-alkylation reaction between amines and alcohols, the electronic state of the active site of precious metals is an important factor affecting the formation, stability, and decomposition of the intermediate state of metal hydrides. The alkali metal cations in zeolite can be regarded as an electronic additive to modulate the electronic state of neighboring precious metals, which is conducive to regulating the activity of dehydrogenation and hydrogen transfer in the hydrogen transfer amination of alcohols.
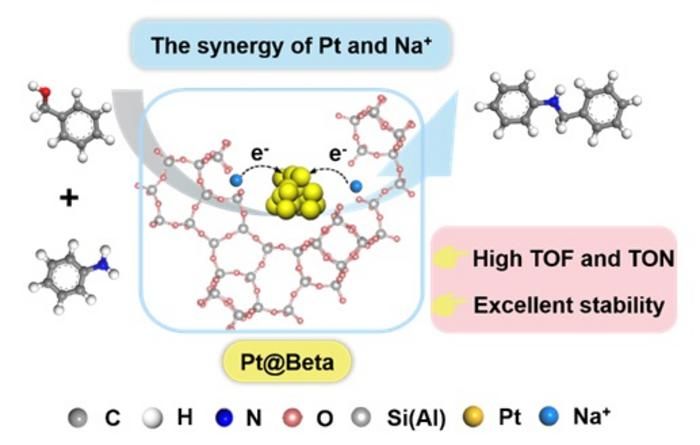
N-alkylamines synthesis through N-alkylation of amines with alcohols catalyzed by Beta zeolites encapsulated Pt nanoparticles
©Science China Press
Recently, Prof. Jun Wang and Prof. Yu Zhou team reported the synthesis of Pt@Beta catalyst with adjustable Si/Al ratio and Na+ contents for the N-alkylation of amines and aromatic alcohols by a one-step hydrothermal synthesis of Pt nanoparticles (NPs) in the crystal structure of BEA zeolite via an acid cohydrolysis route. Under solvent-free conditions, Pt@Beta performed excellent catalytic properties, and conversion number (TON) and conversion frequency (TOF) up to 6176 and 3390 h-1, respectively, and also has stable reusability and wide substrate tolerance. The reaction path and catalytic mechanism were revealed through kinetic analysis and in-situ characterization experiments. Na+ in BEA zeolite regulated the electronic state of Pt NPs, resulting in a moderate intensity Pt–H intermediate state during the reaction process, effectively balancing the two key steps of dehydrogenation and hydrogen transfer, thus achieving an efficient synergistic catalytic process.
Original publication
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Methyl-K
Category:Mu-opioid_agonists
Valerian_(herb)
Category:Biofuel_producers
Fesoterodine
Strain_(chemistry)
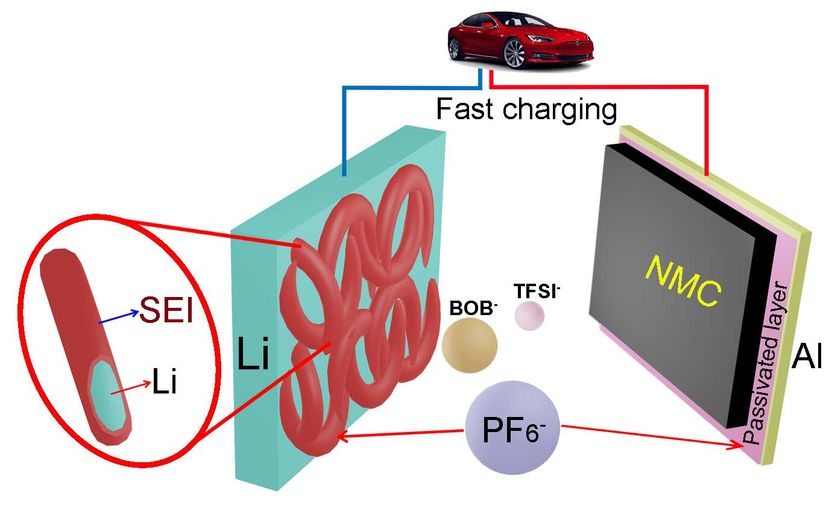
Tweaking electrolyte makes better lithium-metal batteries
Penfluridol
Generalized_epilepsy_with_febrile_seizures_plus