New ammonia reaction could enable sustainable nitrogen source
New system for activation and catalytic transfer of ammonia - catalysis based on main group elements
A major goal of chemistry is to produce amines from ammonia and unsaturated hydrocarbons in a simple way. Moreover, during catalytic addition, in which ammonia is activated and then transferred, no waste is produced, making it particularly sustainable. Researchers at the Karlsruhe Institute of Technology (KIT) have now come a step closer to achieving this goal. They have developed a system for the activation and catalytic transfer of ammonia that is not based on transition metals but on a compound of main group elements.
The molecule ammonia (NH3), a compound of nitrogen and hydrogen, is one of the most widely produced chemicals in the world and forms the starting material for the production of many other nitrogen-containing compounds. If it were possible to produce amines simply by adding ammonia to unsaturated hydrocarbons, chemistry would have made a decisive breakthrough. This is because amines, organic derivatives of ammonia, are in demand in many different areas: They serve, for example, as building blocks for agrochemicals and pharmaceuticals, as well as for washing-active substances, dyes, lubricants and coatings. Amines are also used as catalysts in the production of polyurethanes. Another important application is gas scrubbing in refineries and power plants.
By breaking the strong bond between nitrogen and hydrogen, known as activation, the ammonia molecule can, at least in theory, be transferred to other molecules such as unsaturated hydrocarbons. For example, the transfer of ammonia to the alkene ethylene, an important substance in the chemical industry, would produce ethylamine. Chemists refer to this addition as hydroamination. However, ammonia and ethylene do not just react with each other - a catalyst must mediate the reaction. However, common and transition metal-based catalysts have the disadvantage that they themselves react with ammonia, rendering them inactive. "The hydroamination of non-activated alkenes with ammonia is therefore considered a great challenge, the Holy Grail of catalysis, so to speak," explains Professor Frank Breher, research group leader of the Molecular Chemistry Department at the KIT Institute of Inorganic Chemistry (AOC).
Activation and catalytic transfer of ammonia
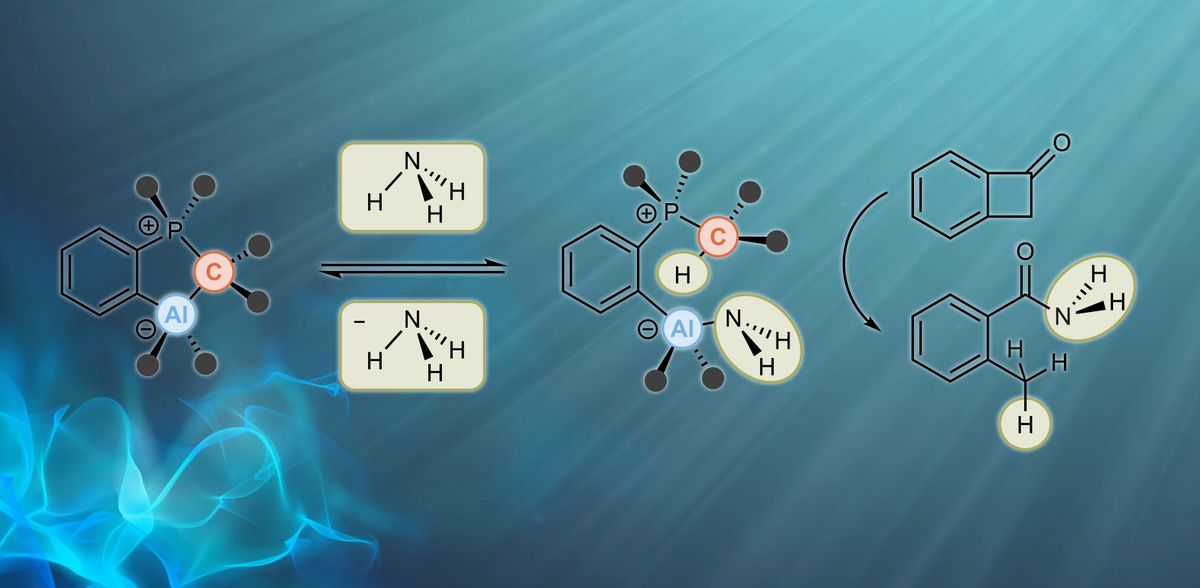
Professor Frank Breher and Dr. Felix Krämer from KIT's AOC, supported by researchers from the University of Paderborn and the Complutense University of Madrid, have now come a big step closer to achieving this challenging goal. "We have developed a system for the activation of ammonia that is not based on transition metals but on main group elements. No waste is generated in the 'atom-economic' process of activation and in the subsequent transfer of ammonia - this is of course particularly interesting from the point of view of sustainability," says Breher. The researchers now report on their work in the journal Nature Chemistry.
The team generated a so-called frustrated Lewis pair (FLP), consisting of an acid as an electron pair acceptor and a base as an electron pair donor. The two usually react with each other to produce an adduct. If adduct formation is prevented or at least limited, a frustrated situation results, so to speak, and the molecule readily reacts with small molecules such as ammonia. "However, it is crucial to dampen the reactivity in such a way that the reaction with small molecules is as reversible as possible, because only then is it possible to use such an FLP in catalysis. We succeeded in doing this for the first time using ammonia as a substrate," reports Breher. The researchers showed that the title compound readily reacts thermoneutrally with non-aqueous ammonia and reversibly cleaves the nitrogen-hydrogen bond of ammonia at room temperature. In addition, their publication is the first to present NH3 transfer reactions mediated by a main group element-based catalyst. "Although we have so far only converted activated substrates and not yet unsaturated hydrocarbons, we have come significantly closer to the 'dream reaction,'" Breher says. "We expect that our first-ever proof of principle will spur further work on the use of N-H-activated ammonia as a readily available and sustainable nitrogen source."
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Category:Women_chemists
'Fuzzy' fibers can take rockets' heat
Menkes_disease
Category:Chemical_companies
Glutathione_S-transferase
Eosinophilia-myalgia_syndrome
List_of_fatty_acid_metabolism_disorders
Oculocerebrorenal_syndrome
3-Methylcrotonyl-CoA_carboxylase_deficiency
Magnesium_deficiency_(medicine)
Organic_compound