Anode Models for Green Hydrogen Production
Advancement of green H2 production
Researchers from the Interface Science Department at the Fritz Haber Institute of the Max Planck Society conducted experiments using atomically defined model pre-catalysts to unveil intricate details of the electrocatalytic water splitting reaction, targeting the advancement of green H2 production.
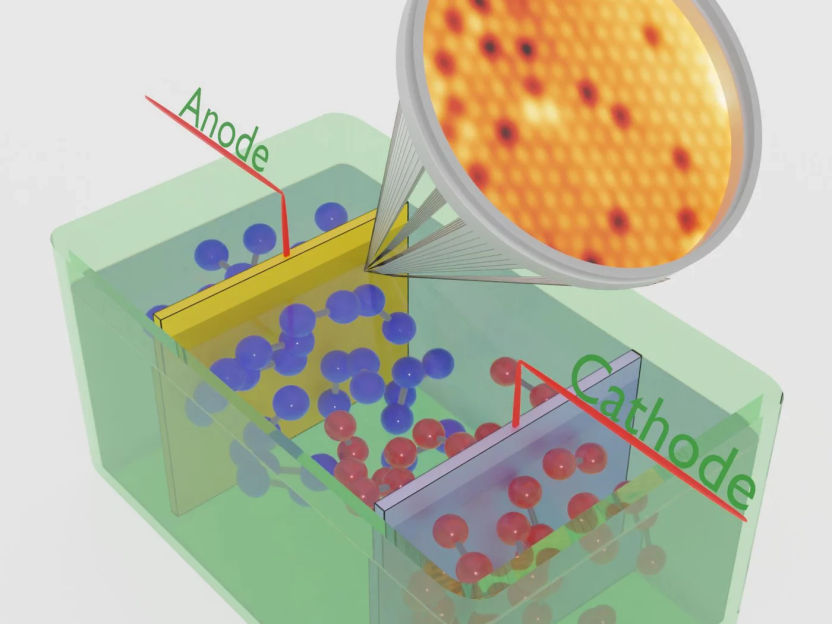
Schematic representation of an electrolyser with a crystalline anode
© FHI
The ongoing climate change poses a serious threat to humanity, affecting everybody’s life and necessitating measures to implement a more sustainable energy economy. The production of ‘green’ energy is a crucial ingredient. However, energy production must be accompanied by economic storage and transport methods. ‘Green’ hydrogen (H2) serves both as a storage medium and a means for transport, also when converted into other useful industrial products or energy carriers such as ammonia. It can be produced by electrolysis via decomposition of water molecules with ‘green’ electrical energy. In the electrocatalytic cell, molecular hydrogen is generated at the cathode, while the anode produces molecular oxygen (O2).
The O2 production at the anode is a complex multistep process, which makes it challenging to design energy-efficient anodes. As a result, most water-splitting research focuses on the anode rather than on the cathode. In real electrolysers, anodes possess intricate chemical compositions and morphologies, impeding the fundamental understanding of electrolysis processes which is very much needed for their subsequent optimization. Relevant data may be challenging to find, akin to a needle in a haystack. To address this, scientists in the Interface Science Department at the FHI have implemented an experimental approach substituting the complex anode with a simpler model pre-catalyst system.
In this approach, the anode pre-catalyst is a well-defined crystalline thin oxide film, allowing for controlled variations in its initial composition and structure. To ensure purity, the anodes are prepared under ultra-high-vacuum conditions, and all subsequent studies are conducted in the same experimental characterization system without exposing the samples to ambient air. This stringent methodology safeguards the anode from contamination throughout the experiment, preventing any adverse effects on the experimental data. Knowing the anode properties in atomic detail is a central aspect of the method. The primary focus is to investigate central aspects of water splitting catalysis, including mechanistic microscopic details of the O2 formation reaction, the active sites, electrode aging, and the role of the anode's surface structure and composition for the water-splitting performance. More specifically, it is well known in the literature that an oxyhydroxide layer is formed on the catalyst surface under operando conditions, but the characteristics of this layer and the optimum structure, thickness and composition are yet unknown. It is however recognized that there is a unifying structural transformation taking place during O2 production, regardless of the initial pre-catalyst structure. On the other hand, and as it is described in the present contribution, the specific characteristics of the pre-catalyst anode determine the transformation that takes place during operation and ultimately the long-term activity and stability of the electrocatalyst.
It is well-known that adding iron to cobalt oxide anodes significantly enhances their performance, although the underlying mechanism is still under discussion. Gaining a comprehensive understanding of the specific role of iron addition is crucial for optimizing water splitting processes. In pursuit of this goal, we conducted a study on crystalline mixed thin film oxide anodes, exploring various Co:Fe ratios. The flat and well-defined anode structure allowed us to establish a quantitative relationship between the oxide's composition, structure, and O2 formation performance, making the beneficial effect of iron addition evident. Stability studies further revealed performance improvements attributed to iron dissolution, eventually converging the catalyst towards a stable highly active anode.
The study addresses two pertinent aspects of water-splitting technology, focusing on minimizing costs associated with electrolyser fabrication and operation. Keeping these costs low by moving towards the alkali reaction conditions and Earth-abundant materials is of crucial relevance for a widespread implementation of a H2-based energy economy. Present electrolyser technology uses rare metals, iridium, and platinum for energy-efficient electrolysis. Replacement of these costly metals by the cheaper cobalt and iron-based oxides would reduce the overall water-splitting cost, increasing the economic attractivity of this process. Electrical efficiency is another crucial cost consideration, relying on the electrode's chemical composition and morphology. This study aims to enhance our understanding of structure-reactivity relationships for a rational electrocatalyst design.
































































