Watching a Bimetallic Catalytic Surface in Action
Such insight on working catalysts is only possible to obtain using state-of-the-art experimental techniques under reaction conditions
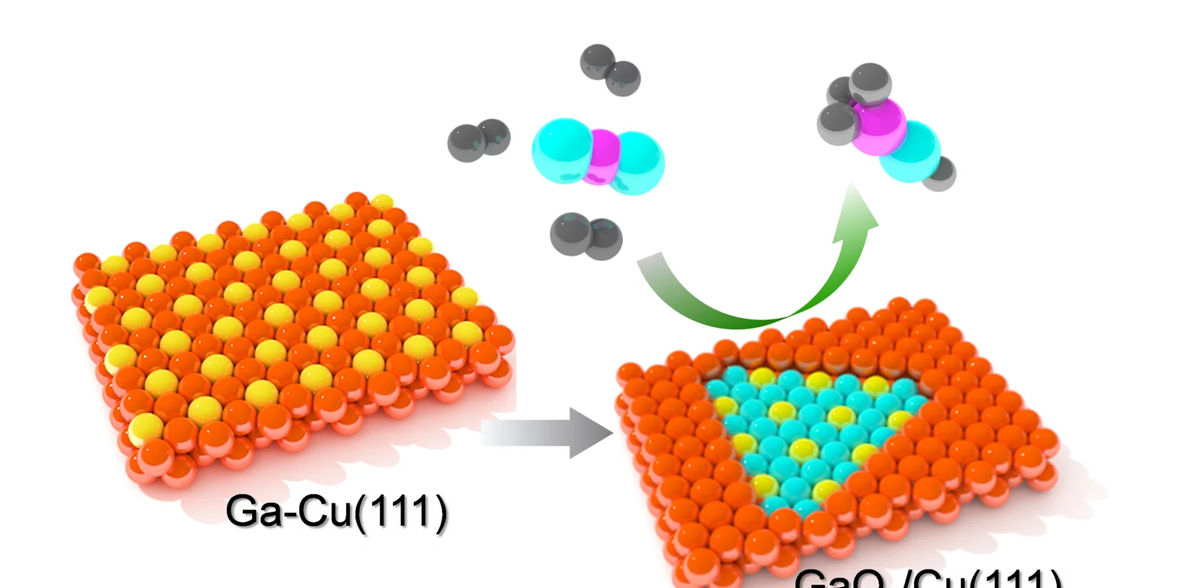
A team of researchers from the Department of Interface Science at the Fritz Haber Institute of the Max Planck Society addressed the question: what happens to a Ga-promoted Cu surface under reaction conditions required for the synthesis of methanol? They found complex structural transformations of this bimetallic catalyst that might change the common view on the catalytically active surface structure.
Hydrogenation of CO2 to methanol occurs with high efficiency on famous Cu/ZnO/Al2O3 catalysts at high pressures, i.e. 50 - 100 bar. However, this synthesis does not only bring along safety risks and a high energy consumption, but also limits the CO2 concentration in the gas feed in order to maintain high selectivity. Therefore, a new class of catalysts for low pressure methanol synthesis is highly desirable, also for future development of small-scale devices using solar-generated hydrogen at ambient pressure.
It has recently been discovered that intermetallic compounds and alloys containing Ga show good catalytic performance even at atmospheric pressures. However, the promotional role of Ga in these catalysts is still poorly understood, primarily because of the lack of information about the surface structures of the catalysts. In this respect, studies using surface-sensitive techniques applied to well-defined model catalysts under reaction conditions can provide key information that will aid our understanding of the dynamic nature of the active sites, reaction intermediates, and ultimately the reaction mechanism.
A team of researchers from the Department of Interface Science at the Fritz Haber Institute took advantage of laboratory-based Near Ambient Pressure X-ray Photoelectron Spectroscopy (NAP-XPS) and Scanning Tunneling Microscopy (NAP-STM), to monitor in situ the structural and chemical evolution of Ga-Cu bimetallic surfaces in the CO2 hydrogenation reaction. They observed temperature- and pressure-dependent de-alloying of the bimetallic surface resulting in Ga-oxide islands embedded into the Cu surface. Although the oxide phase showed a stoichiometry close to Ga2O3, i.e., the most stable Ga-oxide, it actually forms an ultrathin layer. The promotional effect of metals like Ga, which are prone to oxidation, is often discussed within structure models where a bulk oxide is placed on top of the metal surface and the corresponding reaction mechanism involves spillover of intermediate species at the interface. The present study clearly demonstrated that: (i) Ga-oxide is embedded into the metal surface; and (ii) the Ga-oxide islands are ultrathin, most likely of “monolayer” thickness. The reaction -induced formation of ultrathin Ga-oxide layer on metal surfaces is also anticipated for Ga-containing intermetallic compounds. Importantly, such two-dimensional oxide films are very different from their bulk counterparts in terms of structure and reactivity. Therefore, the GaOx/Cu interface formed under CO2 hydrogenation reaction conditions may expose catalytically active sites never considered for this reaction before. Such information would be impossible to obtain using bulk-sensitive techniques commonly employed for the characterization of powder catalysts.
The results of this study, carried out within the framework of the CATLAB project and also supported by the Alexander von Humboldt Foundation, which was just published in Nature Communications, shed light on the complex surface structure of Ga-containing catalytic systems. Such insight on working catalysts is only possible to obtain using state-of-the-art experimental techniques under reaction conditions. Only by establishing the atomic structure of the Ga-oxide layer(s) and its interface to the transition metal under working conditions can one bring insight into the reaction mechanism of this methanol synthesis catalyst.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.