Light regulates structural conversion of chiral molecules
The conversion is relevant e.g. for the preparation of drugs
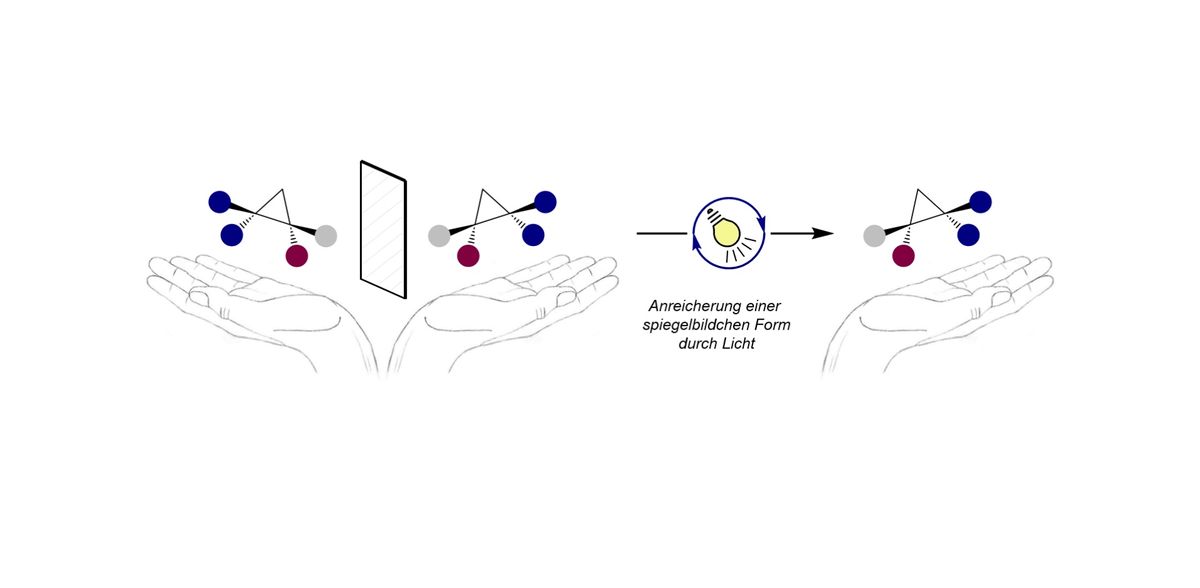
A team of chemists from the University of Münster developed a novel concept in which a mixture of molecules that behave like mirror images is converted to a single form. To this end, they use light as external energy source. The conversion is relevant e.g. for the preparation of drugs. The study is now published in the journal “Nature”.
Just like our hands, certain organic molecules relate to each other like an image and its reflection - a phenomenon that chemists call "chirality" or "handedness". The two mirror images of the same molecule, namely both enantiomers, often possess different biological properties. For example for drug discovery, many times only one of the structures is relevant. However, chemical synthesis methods often create a 1:1 mixture of both forms. Therefore, the selective conversion of these mixtures into one selected form is of great importance. A team of researchers from the Institute of Organic Chemistry and from the Center for Multiscale Theory and Computation at the University of Münster led by Prof. Ryan Gilmour and Prof. Johannes Neugebauer developed a novel concept in which this conversion is enabled by light as an external energy source. The study is now published in the journal “Nature”.
The researchers apply an aluminium complex, that is activated by light, as catalyst to selectively convert a mixture of molecules that behave like mirror images to a single form. The reaction process was investigated experimentally and computationally. The detailed computer-based analyses contributed significantly to the understanding of the underlying processes. The new paradigm impresses with its operational simplicity and broad applicability, as the aluminium complex used is a common catalyst for chemical reactions driven by heat. Translation to light-mediated processes is now envisaged to enable a plethora of new reactivities with great spatial control.
Achieving spatial control in light-mediated reactions is one of the main challenges in contemporary organic chemistry. To this end, usually two distinct catalysts are employed in one reaction: a photocatalyst, that initiates the reactivity, operates in concert with a second catalyst that controls the spatial arrangement of the molecules. Contrarily, the successful integration of both functions in a single catalyst structure was so far only achieved by incorporation of tailored recognition motifs in the catalyst and substrate structures. In this work, the groups present a catalyst that regulates reactivity and selectivity simultaneously. It binds to simple ketones, a functional group that is prevalent in organic molecules, circumventing the need for tailored components. Furthermore, the catalyst is based on earth-abundant aluminium, which is cheaper that the transition metals that are commonly found in photocatalysts.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.