The trio - nickel, palladium, and platinum - for enhanced hydrogen evolution
According to data from the Ministry of Land, Infrastructure and Transport, there were approximately 30,000 hydrogen-powered vehicles registered by 2022, representing a threefold increase compared to 2018. However, the country only has 135 hydrogen fueling stations. In order to enhance the accessibility of hydrogen-powered vehicles and establish hydrogen as a viable energy source, it becomes imperative to reduce the cost of hydrogen production, thereby achieving economic feasibility. To achieve this goal, maximizing the efficiency of electrolysis-hydrogen evolution, the process responsible for producing hydrogen from water, become crucial.
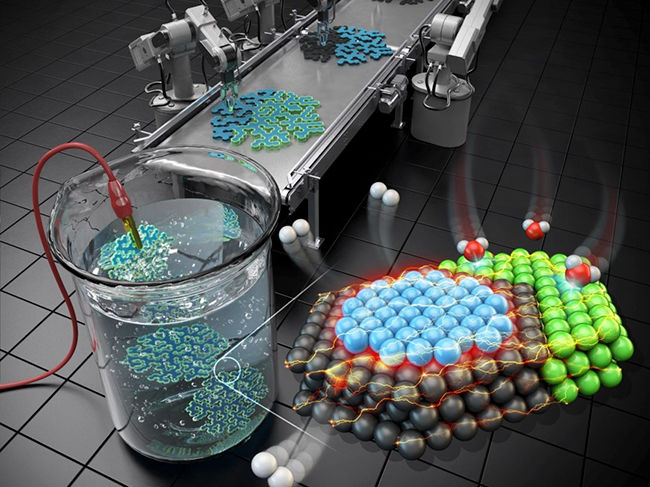
Illustration of the mechanism of the three-metal hybrid nanocatalyst for hydrogen evolution
POSTECH
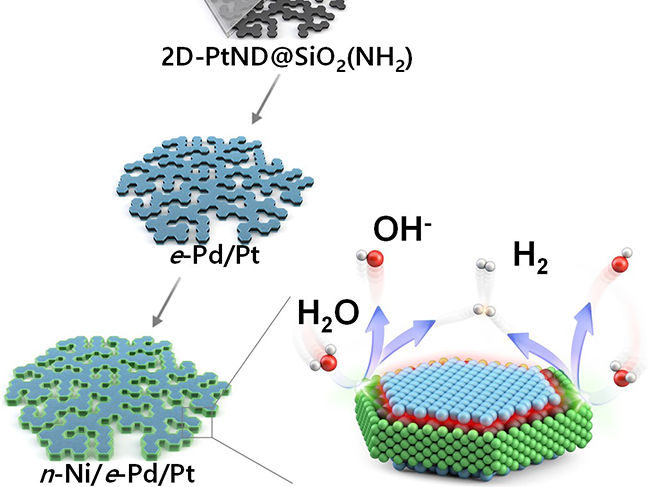
Diagram of the three-metal hybrid catalyst’s synthesis and hydrogen evolution
POSTECH
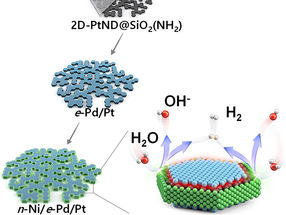
Recently, a team of researchers comprising Professor In Su Lee, Research Professor Soumen Dutta, and Byeong Su Gu from the Department of Chemistry at Pohang University of Science and Technology (POSTECH) achieved a significant improvement in production efficiency of hydrogen, a green energy source, through the development of a platinum nanocatalyst. They accomplished this feat by depositing two different metals in a stepwise manner. The findings of their research were published in Angewandte Chemie, the esteemed journal which focuses on the field of chemistry.
Depositing distinct materials selectively on specific locations of a catalyst surface, whose size is in the nanometer range, poses substantial challenges. Unintended depositions may block the catalyst’s active sites or interfere each other’s functions. This predicament has prevented the simultaneous deposition of nickel and palladium onto a single material. Nickel is responsible for activating water splitting while palladium facilitates the conversion of hydrogen ions into hydrogen molecules.
The research team developed a novel nano reactor to finely control the location of metals deposited onto a 2D flat nanocrystal. Additionally, they devised a nano-scaled fine deposition process, enabling the coverage of different facets of the 2D platinum nanocrystal with different materials. This new approach led to the development of “platinum-nickel-palladium” three-metal hybrid catalyst material achieved through consecutive depositions that selectively cover the flat surface and the edge of the 2D platinum nanocrystal with palladium and nickel nano thin films respectively.
The hybrid catalyst featured distinct nickel/platinum and palladium/platinum interfaces positioned to facilitate the water splitting and hydrogen molecule generation processes respectively. Consequently, the collaborative occurrence of these two different processes significantly boosted the effectiveness of electrolysis-hydrogen evolution.
The research outcomes revealed that the three-metal hybrid nano catalyst exhibited 7.9-fold increase in catalytic activity compared to the conventional platinum-carbon catalyst. Moreover, the novel catalyst demonstrated significant stability, maintaining its high catalytic activity even after a prolonged 50-hour reaction time. This resolved the issue of functional interferences or collisions between heterointerfaces.
Professor In Su Lee who led the research expressed his optimism by stating, “We have successfully developed harmonious heterointerfaces formed on a hybrid material, overcoming the challenges of the process.” He further added, “I hope the research findings will find widespread application in the development of catalytic materials optimized for hydrogen reactions.”