New glass with supreme toughness
Researchers at the University of Bayreuth, together with partners in China and the USA, have produced an oxide glass with unprecedented toughness. Under high pressures and temperatures, they succeeded in paracrystallizing an aluminosilicate glass: The resulting crystal-like structures cause the glass to withstand very high stresses and are retained under ambient conditions. paracrystallization thus proves to be a promising process for producing extremely break-resistant glasses. In "Nature Materials", the researchers present their findings, in which the German Electron Synchrotron (DESY) in Hamburg also participated.
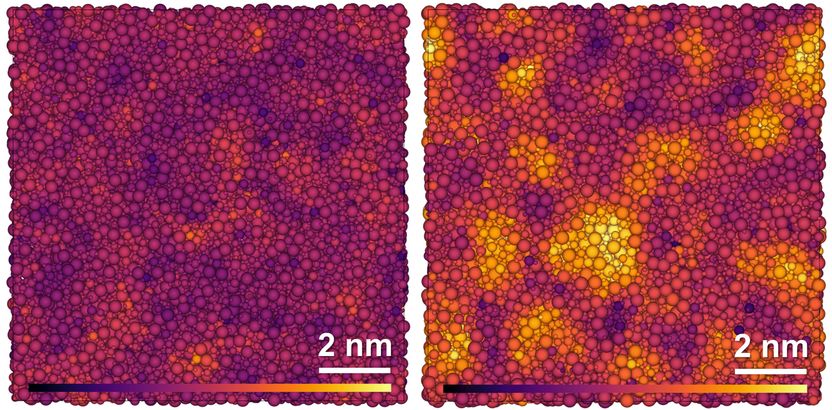
Simulated structure of glassy (left) and paracrystalline (right) grossular. The atoms of the elements oxygen, silicon, aluminum and calcium (from small to large) are coloured lighter the higher the degree of order in the surrounding structure.
Hu Tang
In many respects, glass is an attractive material for modern technologies. However, its brittleness, which easily leads to cracks and fractures, limits its potential applications. Research approaches to highly increase the toughness of glass while retaining its advantageous properties have largely failed to produce the desired results. The new approach presented in "Nature Materials" starts with oxide glasses which have a rather disordered internal structure and are the most widely commercially utilized glass materials. Using aluminosilicate, which contains silicon, aluminum, boron and oxygen, as an example, the research team in Germany and China has now succeeded in giving it a new structure. To this end, they employed high-pressure and high-temperature technologies at the Bavarian Research Institute of Experimental Geochemistry and Geophysics (BGI) of the University of Bayreuth.
At pressures between 10 and 15 gigapascals and a temperature of around 1,000 degrees Celsius, the silicon, aluminum, boron and oxygen atoms grouped together to form crystal-like structures. These structures are called "paracrystalline" because they differ significantly from a completely irregular structure, but they do not approach the clear regular structure of crystals. Both empirical analyses using spectroscopic techniques and theoretical calculations clearly showed this intermediate state between crystal structures and amorphous irregularity. Even after a drop in pressure and temperature to normal ambient conditions, the paracrystalline structures in the aluminosilicate glass remain. The penetration of the glass with these structures results in the toughness of the glass being many times higher than before paracrystallization. It now reaches a value of up to 1,99 ± 0,06 MPa (m)¹/². This is a toughness never before measured for oxide glasses. At the same time, the transparency of the glass is not seriously affected by the paracrystalline structures.
The researchers explain the extraordinary strengthening of the glass by the fact that forces acting on the glass from outside, which would normally lead to breakage or internal cracks, are now primarily directed against the paracrystalline structures. They dissolve areas of these structures and transform them back into an amorphous, random state. In this way, the glass as a whole acquires greater internal plasticity, so that it does not break or crack when it is exposed to these or even to stronger forces.
"Our discovery highlights an effective strategy for developing highly damage-tolerant glass materials, which we plan to pursue with our research in the coming years," said Dr. Hu Tang, first author of the new study. "The increase in toughness due to paracrystallization shows that structural changes at the atomic level can have a significant impact on the properties of oxide glasses. At this level, there is great potential for optimizing glass as a material that is far from being exhausted," adds Prof. Dr. Tomoo Katsura of the Bavarian Research Institute of Experimental Geochemistry and Geophysics.
Original publication
Original publication
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Baking blueberries changes their polyphenol content

European Chemical Transport Association (ECTA) - Brüssel, Belgium

KAINDL Technischer Industriebedarf Gesellschaft m.b.H. - Leonding, Austria




























































