Potentially producing carbon-neutral fuels
Chemists achieve sustainable solar energy conversion with artificial photocatalysis
Converting solar energy into carbon-neutral fuels is a promising approach to reduce our dependence on fossil fuels and combat climate change. Taking examples from nature, plants and other photosynthetic organisms use sunlight to create energy-rich compounds from water and carbon dioxide (CO2) through a complex biochemical process that take places within specialised structures called chloroplasts. However, the efficiency of this natural process is limited by metabolic pathway that have a reflectively low effectiveness in converting sunlight into useful energy. While artificial photocatalytic cycles have demonstrated higher intrinsic efficiencies, they typically rely on pure or highly concentrated CO2 and organic media to prevent degradation of the catalyst caused by water or protons.
Research teams led by Professor David Lee PHILLIPS from the Department of Chemistry of The University of Hong Kong (HKU), Professor Lili DU at Jiangsu University (HKU PhD Alumna), Professor Ruquan YE at the City University of Hong Kong and Professor Jia TIAN at the Shanghai Institute of Organic Chemistry have developed a remarkable and environmentally friendly system that can effectively harness light energy for the photocatalytic process. This artificial system is highly-stable and recyclable, and it does not rely on precious metals, making it more economically viable and sustainable. The research findings are recently published online in the scientific journal Nature Catalysis.
Background and Achievement
In nature, organisms use a process called ‘hierarchical self-assembly’ to optimise light harvesting. During the process, they organise photocatalytic components within a tailored environment provided by lipid or protein-based scaffolds. By achieving high stability, selectivity, and efficiency, photosynthesis relies on the high surface area and precise spatial control of chromophore molecules and catalytic centres through self-assembly, which offers a design principle for highly efficient artificial photocatalytic systems.
Recent studies have demonstrated using vesicles and micelles formed by co-assembly of natural lipids or synthetic surfactants with photocatalytic species. These structures act as micro-reactors, mimicking the environment of cell membranes. However, replicating the natural light-harvesting super complexes via synthetic pathways is hard to implement and far from cost-effective.
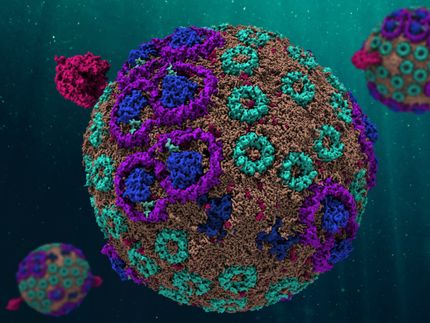
With full appreciation for the current efforts and the challenges, the HKU team and their collaborators have designed a self-assembling artificial spherical chromatophore nanomicelle system in water inspired by the photosynthetic apparatus of Rhodobacter sphaeroides, a type of bacteria that is commonly found in soil and freshwater, which has a special structure called ‘spherical light-harvesting chromatophore’. This structure acts as a light sensor and possesses a remarkable ability to efficiently transfer energy from sunlight via a unique effect called ‘the spherical antenna effect’, created by circular arrangements of specific molecules on the surface of the chromatophore. This allows the bacteria to capture and utilise sunlight effectively for their energy needs.
This artificial system mimics the bacteria's spherical light harvesting chromatophore and comsists of tiny spherical structures called nanomicelles that are self-assembled in aqueous solutions. These nanomicelles serve as the building blocks of the system. The system utilises modified molecules and light-absorbing compounds known as ‘aramid-linker enhanced Zn porphyrin amphiphiles’, which interact with a Co catalyst through electrostatic forces, leading to a unique hierarchical assembly. Hence, this assembly is induced by ‘spherical antenna effect’, and enhances the system to capture and alight energy for photocatalytic processes.
The hierarchical self-assembly of the system offers a promising bottom-up strategy to create a precisely controlled artificial photocatalytic system with high stability and efficiency based on cheap and Earth-abundant elements rather than expensive precious metals.
Professor David Phillips said: "Our research has the potential to advance renewable energy by replicating nature's efficient light-harvesting mechanisms. This could lead to sustainable solutions for our energy needs and the production of carbon-neutral fuels, contributing to a greener future."
Professor Lili Du said: "The self-assembling artificial system is a significant step towards unlocking the full potential of solar energy conversion. Improving photocatalytic efficiency and stability can overcome limitations and create a cleaner, more sustainable energy landscape. This research offers promising practical applications in fuel production, carbon capture, and environmental remediation."
Original publication
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.