Microbes powered by electricity
How bacteria use electricity and carbon dioxide to produce useful chemicals
In microbial electrosynthesis, microorganisms use CO2 and electricity to produce alcohol, for example. How this process works biologically, however, has only been speculated about until now. Researchers at the Leibniz Institute for Natural Product Research and Infection Biology (Leibniz-HKI) have now been able to confirm experimentally for the first time that the bacteria use electrons from hydrogen and can produce more chemical substances than previously known.
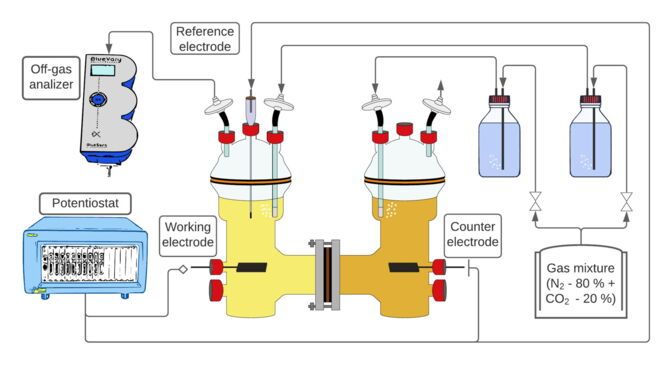
Schematic representation of the experimental setup: The bacterial culture grows in one of the containers, electricity and CO2 are supplied. A second container is used for the electrochemical counter-reaction; oxygen is produced here.
Santiago Boto/Leibniz-HKI
Microbial electrosynthesis is a promising technology against the backdrop of climate change and the energy transition: it can bind carbon dioxide, produce ethanol and other organic compounds that can be used as fuel, and thus store excess electricity. Nevertheless, the technology, which has been known for more than a decade, has so far failed to achieve any significant breakthrough towards commercialization.
According to Miriam Rosenbaum, head of the Bio Pilot Plant at Leibniz-HKI, this is mainly because "the biology behind the process has so far been regarded as a kind of black box." The biochemist, who holds the Chair of Synthetic Biotechnology at Friedrich Schiller University in Jena, has long been dedicated to the question of what exactly happens during microbial electrosynthesis (MES).
Her team has now made a breakthrough in precisely this area: The researchers were able to show that bacteria do not directly absorb the electrons supplied by electric current, but instead use hydrogen to transfer the electrons. This had long been suspected as a possibility, but until now no one had provided experimental proof. They also found that the method could produce even more useful chemicals than previously thought and optimized the process for the highest possible yields.
Controlled conditions
In MES, electricity is applied to an aqueous nutrient solution containing microorganisms, and carbon dioxide is added at the same time. The microorganisms use the electricity and carbon to produce organic compounds such as ethanol or acetate. To do this, they use the supplied electrons - but it was previously unclear how.
"There was one study that assumed that the microbes used the electrons directly," Rosenbaum says. However, this hypothesis was not proven. Rosenbaum thought it more likely that the microbes were using hydrogen for their biosynthesis. That's because when electricity and carbon dioxide are applied, what happens is the same as in classical electrolysis: Water is split into hydrogen and oxygen.
"Until now, no one has really measured hydrogen directly in the system," explains Santiago Boto, lead author of the study. He therefore set up the MES reactor so that he can precisely control all parameters. To do this, he uses a pure culture with the bacterium Clostridium ljungdahlii in a range of different concentrations. In addition, he can control the electric current flow and measure the hydrogen produced at the electrode and the hydrogen escaping from the liquid using microsensors.
"With our design, we were able to gather several pieces of evidence that the bacteria were using hydrogen," Boto said. When the concentration of bacteria in the nutrient medium was such that they formed a biofilm on the cathode and little hydrogen was measurable in the electrode environment, the activity of the bacteria was significantly reduced. This also happened when the voltage was not high enough for electrolysis. Only when hydrogen was freely available to planktonic - i.e. free-swimming - bacteria from the electrode did they show high activity.
New biosynthetic pathways uncovered
In this way, the research team was able to optimize voltage and bacterial concentration for the highest possible acetate yields. "We had the highest acetate values achieved to date for a pure culture of bacteria," Boto said. As a side result, he also found that amino compounds were formed that the bacteria do not normally produce. In cooperation with Falk Harnisch from the Environmental Research Center in Leipzig, the work also showed that reactions between the nutrient medium and the cathode, which had also not been described before, occur, apparently accelerating the synthesis process.
The team now wants to optimize the processes even further and specifically explore the previous findings. "Amino compounds are very interesting for the chemical industry, and the bacteria we used are also already in industrial use. We may have thus discovered a new production method for such chemicals," Boto said. Overall, the results should help make MES commercially viable. "I expect that we will see a strong upswing in this technology in the coming years when we finally focus on biology as well," Rosenbaum said. The Bio Pilot Plant is collaborating on this and partnering with process engineers to develop larger reactors for MES.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.