Cryo-imaging lifts the lid on fuel cell catalyst layers
First insights into the nanostructure of platinum catalyst layers
Thanks to a novel combination of cryogenic transmission electron tomography and deep learning, EPFL researchers have provided a first look at the nanostructure of platinum catalyst layers, revealing how they could be optimized for fuel cell efficiency.
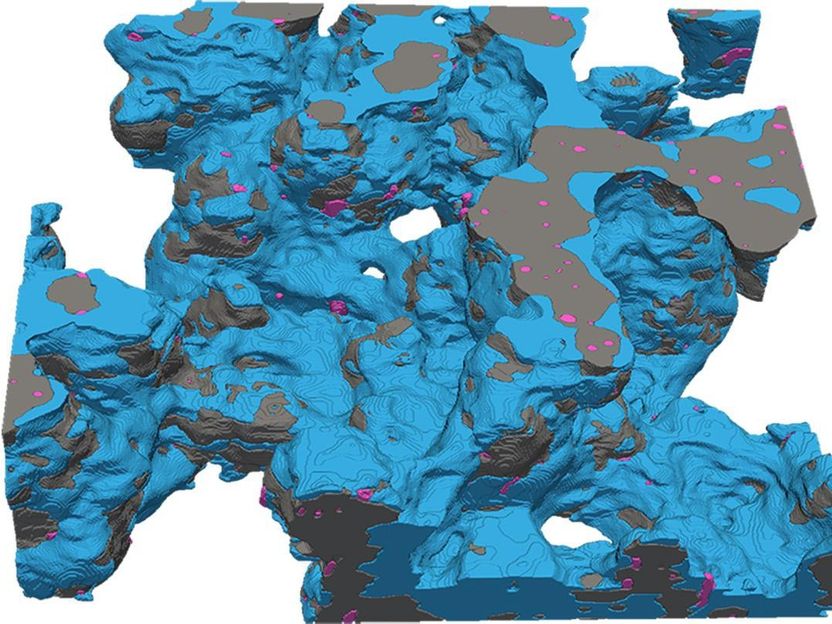
In one of the lab's images, platinum nanoparticles are shown in pink
© INE EPFL
Proton-exchange membrane fuel cells (PEMFC), which are being developed for use in electric vehicles, rely on nanoparticles called catalysts to trigger electricity-producing reactions between hydrogen and oxygen. Most PEMFC catalysts contain platinum – a scarce and precious metal. There is therefore a pressing global need to develop catalysts that can generate the most power while minimizing platinum content.
Manufacturers integrate these catalysts in complex assemblies called catalyst layers. Until now, they had to do so without a detailed picture of the resulting structure, as traditional imaging processes almost always cause some degree of damage.
Vasiliki Tileli, head of the Laboratory for in-situ nanomaterials characterization with electrons in the School of Engineering, has found a way around this challenge. By imaging catalysts and their environment at below-freezing temperatures using cryogenic transmission electron tomography and processing the images with deep learning, she and her colleagues have succeeded in revealing, for the first time, the nanoscale structure of catalyst layers.
“We’re still far away from PEMFCs without platinum, which is very expensive, so in the short term, we need to reduce platinum loading to make this technology viable for mass production. It’s therefore imperative to understand how platinum sits in relation to other materials within the catalyst layer, to increase the surface area contact required for chemical reactions to take place,” Tileli explains.
“That’s why it’s quite an achievement to image these catalysts in three dimensions; before, it was impossible to have the right contrast between the different catalyst layer components.”
The work has recently been published in the journal Nature Catalysis.
Better preservation; higher resolution
During imaging using conventional electron microscopy, delicate catalyst layer samples often become damaged by electron beams, causing materials to shrink or deform. By carrying out the imaging in-situ at cryo-temperatures, Tileli and her team were able to preserve most of the catalyst layer’s morphology. Then, they used a machine learning algorithm to more accurately denoise and classify the images, allowing them to achieve a higher image resolution than had ever previously been possible.
Crucially, the scientists were able to reveal the heterogenous thickness of a porous polymer layer on the catalysts called ionomer. Ionomer thickness strongly influences how well platinum catalysts perform.
“The ionomer must have a certain thickness for the catalytic reactions to happen efficiently. Because we could do a full reconstruction of catalyst layers with limited damage to the structure, we could show, for the first time, how much platinum is covered with ionomer and the thickness of that coverage,” Tileli explains.
Such information could be a gold mine for catalyst manufacturers, who could use it to produce catalysts with more platinum particles that are covered by the right amount of ionomer – and that therefore perform optimally.
“The cryo-aspect is the key component of this study. Ionomers are like proteins: they are soft, and require freezing conditions to stabilize and protect their structure,” Tileli says.
“I think this advanced technique will therefore be useful not just for facilitating the mass manufacturing of PEMFCs through optimized platinum use, but also for many different materials science and energy applications – for example, battery storage, water electrolysis, and energy conversion systems in general.”