How football-shaped molecules occur in the universe
Solving the puzzle of classic buckyballs
For a long time it has been suspected that fullerene and its derivatives could form naturally in the universe. These are large carbon molecules shaped like a football, salad bowl or nanotube. An international team of researchers using the Swiss SLS synchrotron light source at PSI has shown how this reaction works. The results have just been published in the journal Nature Communications.
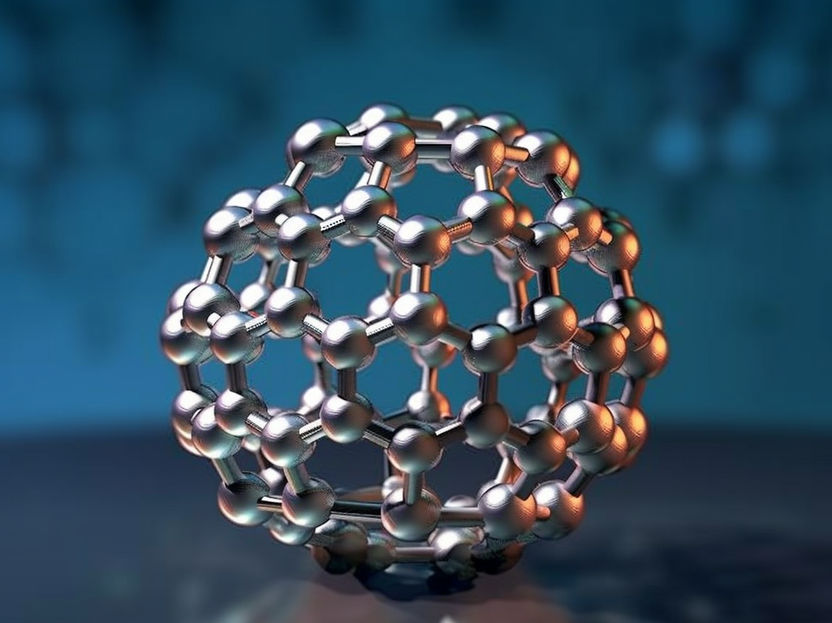
Symbolic image
Computer-generated image
“We are stardust, we are golden. We are billion-year-old carbon.” In the song they performed at Woodstock, the US group Crosby, Stills, Nash & Young summarised what humans are essentially made of: star dust. Anyone with a little knowledge of astronomy can confirm the words of the cult American band – both the planets and we humans are actually made up of dust from burnt-out supernovae and carbon compounds billions of years old. The universe is a giant reactor and understanding these reactions means understanding the origins and development of the universe – and where humans come from.
In the past, the formation of fullerenes and their derivatives in the universe has been a puzzle. These carbon molecules, in the shape of a football, bowl or small tube, were first created in the laboratory in the 1980s. In 2010 the infrared space telescope Spitzer discovered the C60 molecules with the characteristic shape of a soccer ball, known as buckyballs, in the planetary nebula Tc 1. They are therefore the biggest molecules to have been discovered to date known to exist in the universe beyond our solar system.
But how do they actually form there? A team of researchers from Honolulu (USA), Miami (USA) and Tianjin (China) has now completed an important reaction step in the formation of the molecules, with active support from PSI and the vacuum ultraviolet (VUV) beamline of the synchrotron light source Swiss SLS. “PSI offers unique experimental facilities and that’s why we decided to collaborate with Patrick Hemberger at PSI,” says Ralf Kaiser from the University of Hawaii in Honolulu, the leading international researcher in this field.
A mini reactor for fullerene
Patrick Hemberger, a scientist working on the VUV beamline at PSI, has built a mini reactor for observing the formation of fullerene in real time. A corannulene radical (C20H9) is created in a reactor at a temperature of 1,000 degrees Celsius. This molecule looks like a salad bowl, as if it had been dissected from a C60 buckyball. This radical is highly reactive. It reacts with vinyl acetylene (C4H4), which deposits a layer of carbon onto the rim of the bowl. “By repeating this process many times, the molecule would grow into the end cap of a nanotube. We have managed to demonstrate this phenomenon in computer simulations,” explains Alexander Mebel, Professor of Chemistry at Florida International University and one of the authors of the study. But that was not the researchers’ only goal: “We wanted to show that this type of reaction is physically possible,” Ralf Kaiser adds.
The reaction produces different isomers – molecules that all have the same mass, but slightly different structures. With standard mass spectrometry, all these variants produce the same signal. But the outcome is different when using photoelectron photoion coincidence spectroscopy, the method adopted by the team. “With this technique, the structure of the measurement curve allows conclusions to be drawn about each individual isomer,” Patrick Hemberger explains.
Solving the puzzle of classic football-shaped molecules
“The universe contains a wild jungle of molecules and chemical reactions – not all of them can be distinctly classified in the signals from telescopes,” says Ralf Kaiser. We already know from models that both corannulene and vinylacetylene exist in the universe. Now it has been possible to confirm that these molecules actually form the building blocks to fullerene. “That’s why the experiment at PSI is so valuable for us.”
But the successful publication in Nature Communications is not the end of the story. The researchers want to conduct more experiments in order to understand how the classic buckyballs form in the universe, along with the football-shaped fullerene molecules with 60 carbon atoms and the minute nanotubes with even more atoms.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Anti-counterfeiting fluorescent marker developed - Sweet code with laser printing
Leopold_Heinrich_Fischer
Gonadotropin-releasing_hormone
Melanocortin_1_receptor
George_N._Hatsopoulos
Bacillus_Calmette-Guérin
Entecavir

TSKgel HXL Columns | SEC columns | Tosoh
Platelets instead of spheres make screens more economical
Category:Apolipoproteins