Mystery of two-dimensional quasicrystal formation from metal oxides solved
"We are excited to learn whether this special arrangement will produce completely new and useful properties"
The structure of two-dimensional titanium oxide brakes-up at high temperatures by adding barium; instead of regular hexagons, rings of four, seven and ten atoms are created that order aperiodically. A team at Martin Luther University Halle-Wittenberg (MLU) made this discovery in colaboration with researchers from the Max Planck Institute (MPI) for Microstructure Physics, the Université Grenoble Alpes and the National Institute of Standards and Technology (Gaithersburg, USA), thereby solving the riddle of two-dimensional quasicrystal formation from metal oxides. Their findings have been published in the journal "Nature Communications".
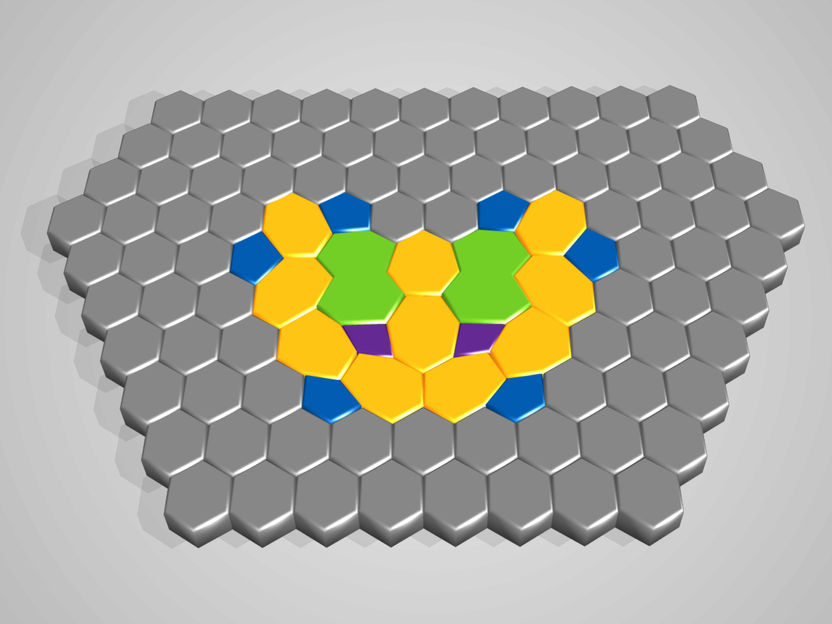
A substructure consisting of rings of different sizes embeds itself seamlessly into a hexagonal structure.
Uni Halle / Stefan Förster
Hexagons are frequently found in nature. The best-known example is honeycomb, but graphene or various metal oxides, such as titanium oxide, also form this structure. "Hexagons are an ideal pattern for periodic arrangements," explains Dr Stefan Förster, researcher inthe Surface and Interface Physics group at MLU’s Institute of Physics. "They fit together so perfectly that there are no gaps." In 2013, this group made an astonishing discovery upon depositing an ultrathin layer containing titanium oxide and barium on a platinum substrate and heating it to around 1,000 degrees centigrade in ultra-high vacuum. The atoms arranged themselves into triangles, squares and rhombuses that group in even larger symmetrical shapes with twelve edges. A structure with 12-fold rotational symmetry was created, instead of the expected 6-fold periodicity. According to Förster, "Quasicrystals were created that have an aperiodic structure. This structure is made of basic atomic clusters that are highly ordered, even if the systematics behind this ordering is difficult for the observer to discern." The physicists from Halle were the first worldwide to demonstrate the formation of two-dimensional quasicrystals in metal oxides.
The mechanisms underlying the formation of such quasicrystals remained puzzling since their discovery. The physicists at MLU have now solved this riddle in collaboration with researchers from the Max Planck Institute for Microstructure Physics Halle, the Université Grenoble Alpes and the National Institute of Standards and Technology (Gaithersburg, USA). Using elaborate experiments, energetic calculations and high-resolution microscopy, they have shown that high temperatures and the presence of barium create a network of titanium and oxygen rings with four, seven and ten atoms respectively. "The barium both breaks up the atomic rings and stabilises them," explains Förster, who heads the joint project. "One barium atom is embedded in a ring of seven, two in a ring of ten." This is possible because the barium atoms interactelectrostatically with the platinum support, but do not form a chemical bond with the titanium or oxygen atoms.
With their latest discovery the researchers have done more than just clarify a fundamental question of physics. "Now that we have a better understanding of the formation mechanisms on the atomic level, we can try to fabricate such two-dimensional quasicrystals on demand in other application-relevant materials like metal oxides or graphene," says Förster. "We are excited to learn whether this special arrangement will produce completely new and useful properties."
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Towards a Greener Space Economy - ISPTech Secures €2M in Pre-Seed Funding for Innovative Spacecraft Propulsion Technology
IChemE confirms new plans for China