Simulations and experiments reveal unprecedented detail about water’s motion in salt water
Researchers captured extremely fast dynamics
In salt water solutions, Water molecules rapidly move around salt ions at a scale of more than a trillion times a second, according to both experiments and simulations led by scientists at New York University and the Sorbonne.
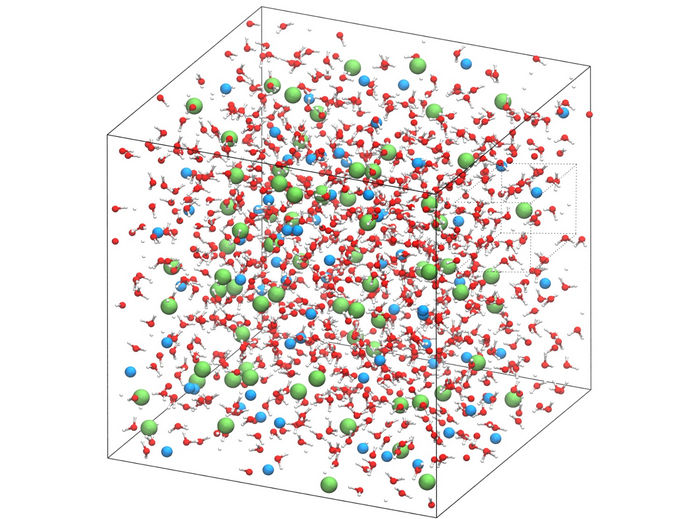
Simulation of salt water solution. Water molecules are shown red and white, chloride ions in green, and in blue, sodium ions.
© Iurii Chubak
“There is more to salt solutions than meets the eye,” said Alexej Jerschow, a professor in NYU’s Department of Chemistry and one of the study’s senior authors. “This was evident when we both measured and modeled the very fast dynamics of sodium chloride ions and surrounding water molecules.”
The findings, published in Nature Communications, will allow researchers to build more reliable models for predicting ion dynamics, which could be used for a variety of scientific endeavors, from improving rechargeable batteries to MRIs.
Ions are ubiquitous and critical to life. Many ions, such as sodium and potassium, are pervasive through the human body and dictate cell viability, nerve signaling, and structural integrity of tissues. How ions interact with solvents plays a critical role as well; for instance, rechargeable batteries rely on the motion of ions through electrolyte solutions.
Ions in a water-based solution are typically surrounded by four to six water molecules, but it is not well understood to what extent these molecules move as a unit and how much motion the water molecules experience. Models used previously have been inadequate in capturing the concerted motion between water and the ions.
To study the movement of salt and water molecules, the researchers used nuclear magnetic resonance (NMR) spectroscopy, a versatile tool that is routinely used to determine the structure of molecules, and combined the experimental data with detailed computer simulations that can model the dynamics around salt ions on an atomic scale.
Testing salt water over a wide range of concentrations and temperatures, and combining experimental data and computer simulations, the researchers observed that water molecules wiggle around the sodium and chloride ions at an extremely rapid pace—more than a trillion times a second. In addition, it was previously assumed that ions move together with surrounding solvent molecules as a unit, but the experiment showed that this is not the case; instead, the water molecules wiggle much faster than the ion-water complex.
“We found excellent agreement between experiment and simulations, which allows us to build reliable models for ion dynamics,” said Jerschow.
“We are now turning to more complex electrolytes and to what happens near solid surfaces, and combining experiments with simulations will again be essential to make progress," said Benjamin Rotenberg of Sorbonne Université and France’s Centre national de la recherche scientifique (CNRS), and the study’s other senior author.
“We anticipate that this work can provide insights in many areas—from medicine to energy storage—that build on a good understanding of ion dynamics in solution,” added Jerschow.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.