Catalyzing ‘net-zero’ green hydrogen from the sun
Chemists discover a fundamental catalyst protonation process to promote solar-driven water-splitting for hydrogen production without CO2 emissions
hydrogen is a promising green energy carrier for a sustainable future. However, it is mostly locked in water. Energy is required to liberate it from water for practical use. Solar energy is abundantly renewable, ideal for direct water-splitting to generate hydrogen using a ‘photocatalyst’. However, despite of considerable effort, practical adoption has been slow due to relatively low efficiency and high cost of the catalyst.
Chemists at HKU discover a fundamental catalyst protonation process to enhance productivity of solar-driven water-splitting for hydrogen by eight times, catalysing green energy without CO2 emissions.
The University of Hong Kong
A research team led by Professor Zheng-Xiao GUO and Professor David Lee PHILLIPS from the HKU-CAS Joint Laboratory on New Materials and the Department of Chemistry of The University of Hong Kong (HKU), has reported the discovery of an important in-situ protonation process that the photodynamics and separation of charge carriers in a photocatalyst, leading to efficient hydrogen generation from water using visible solar light. The process is enabled in an interstitial phosphorus doped carbon nitride structure, with only earth-abundant non-metallic elements, for its cost-effectiveness and high potential for practical applications. The research findings are recently published online in a top scientific journal, Energy & Environmental Science.
Background and Achievement
Extensive research efforts have been devoted to the development of photocatalysts for solar-driven energy conversion with improved activity, efficiency and durability, mostly via: charge separation, transfer and utilisation. However, the complex multi-electron transfer, proton coupling and intermediate dynamics can all influence the photocatalytic pathway, kinetics and efficiency, which have not been well understood. It is thus highly desirable to foster in-depth investigations integrating innovative synthesis design, microscopic and spectroscopic characterisations and atomic simulations at the molecular level.
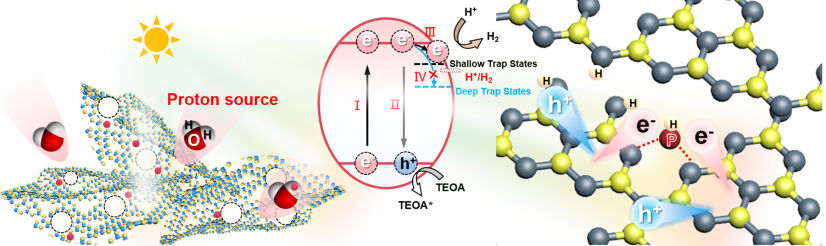
With full appreciation of the current efforts and the challenges in photocatalysis, the HKU team examined the fundamental issues from a different angle and proposed a new fundamental process of a proton-mediated photocatalytic mechanism to enhance the photo-dynamics, charge separation and hence the overall efficiency of an interstitial phosphorus-doped carbon-nitride, g-C3N4. The in-situ proton-mediated mechanism points to a new role of the water molecule, not just as a solvent or reactant but as an effective band-structure modifier of the catalyst in the overall design of effective photocatalytic processes.
In essence, the team has developed an effective atomic heterojunction by porosity-stabilised interstitial P-doping and in-situ protonation to induce shallow trap states, which effectively: a) enhance the lifetime of the excited states and b) restrain undesirable deep charge trapping, leading to efficient water decomposition. For the first time, the team has identified that the in-situ protonation of an interstitially anchored phosphorus in a holey g-C3-xN4 is a very effective structural configuration of the catalyst for highly efficient and stable visible-light hydrogen generation.
‘We expect that our discovery will open up a new line of thinking in the future design of photocatalysts for effective solar energy utilisation, by paying more attention to operando structural dynamism as a viable handle to pump up the conversion efficiency,’ said Professor Zheng-Xiao Guo.
‘Spectroscopic investigations show a colourful world of nanomaterials, and it will cast more light on the mechanistic insights of science and technologies,’ echoed Professor David Lee Phillips.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
High harmonics illuminate the movement of atoms and electrons - Detailed new insights into atomic motions
Diborane
Radiometric_dating

Restriction and authorisation found to drive replacement of harmful chemicals - Replacing harmful chemicals with safer alternatives and greener technologies is strongly driven by regulation
CABB to acquire KemFine Group Oy
Unusual electronic state found in new class of unconventional superconductors - Finding gives scientists a new group of materials to explore to unlock secrets of some materials' ability to carry current with no energy loss
Category:Battery_swapping
HLA-A34