Mild Ammonia Synthesis
Using boron radicals to convert nitrogen to ammonia in solution
ammonia is obtained industrially using the Haber–Bosch process, which requires a lot of energy and hydrogen gas. A much milder approach has now been reported by a team of researchers in the journal Angewandte Chemie. According to this research, reactive boron compounds can efficiently target atmospheric nitrogen and convert it to ammonium chloride after the addition of an acid. This conversion takes place in solution, at room temperature, and without the need for metals or hydrogen gas.

Symbolic image
Computer-generated image
(c) Wiley-VCH

Nitrogen makes up 77% of the air we breathe, and so, in theory, it is virtually infinitely available for ammonia synthesis. However, in practice, it only reacts extremely slowly with other elements. In the Haber–Bosch process, which was developed over 100 years ago, metal catalysts accelerate this sluggish reaction. They activate the nitrogen which is then reacted with hydrogen under high pressure and temperature, giving ammonia.
Ammonia is used industrially for producing nitrate fertilizers. It can also be used as a hydrogen store when hydrogen is used as a source of energy. To date, microbiological methods for nitrogen fixation have been the predominant milder alternative proposed for the Haber–Bosch process. However, exploiting bacteria for biotechnological ammonia production is still quite inefficient.
A team of researchers headed by Nicolas Mézailles of the Université Paul Sabatier, CNRS, in Toulouse, France, have now discovered that reactive boron compounds can very efficiently target and activate molecular nitrogen. The team explained their initial thinking: “We reasoned that the use of high-energy radicals might provide a kinetically and thermodynamically favorable pathway to nitrogen functionalization.”
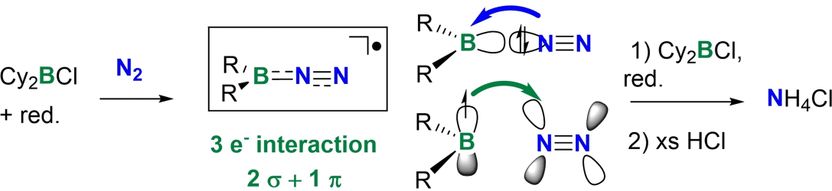
The team’s theoretical calculations then highlighted boron-centered radicals as suitable candidates. The researchers produced these boron-centered radicals by adding a strong reducing agent to organic boron halides. The resulting substances converted molecular nitrogen at room temperature to borylamines, which in turn reacted with aqueous acid to give ammonium chloride.
Mézailles and the team have now described a novel approach to nitrogen fixation in solution using radical compounds. The researchers observed that the boron-centered radicals they produced efficiently broke down the stable triple bond in molecular nitrogen, making it possible to functionalize molecular nitrogen under mild conditions. This radical-based approach opens up further possibilities for ammonia production without having to rely on fossil-based raw materials.
Original publication
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.




























































