International research team creates previously unknown nitrogen compounds
The study exemplifies the great, as yet untapped potential of high-pressure research for nitrogen chemistry
Non-metal nitrides are compounds in which nitrogen and non-metallic elements are linked by covalent bonds. Because of their technologically interesting properties, they have increasingly become the focus of materials research. In the journal "Chemistry – A European Journal", an international team with researchers from the University of Bayreuth presents previously unknown phosphorus-nitrogen compounds synthesized under very high pressures. They contain structural units whose existence could not been empirically proven before. The study exemplifies the great, as yet untapped potential of high-pressure research for nitrogen chemistry.
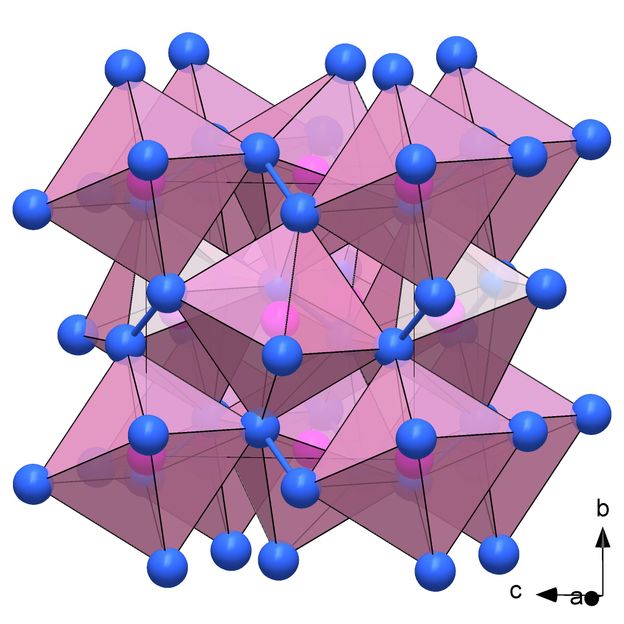
Empirically demonstrated for the first time: the structure of PN₂ consisting of PN₆ octahedra.
(c) Dominique Laniel
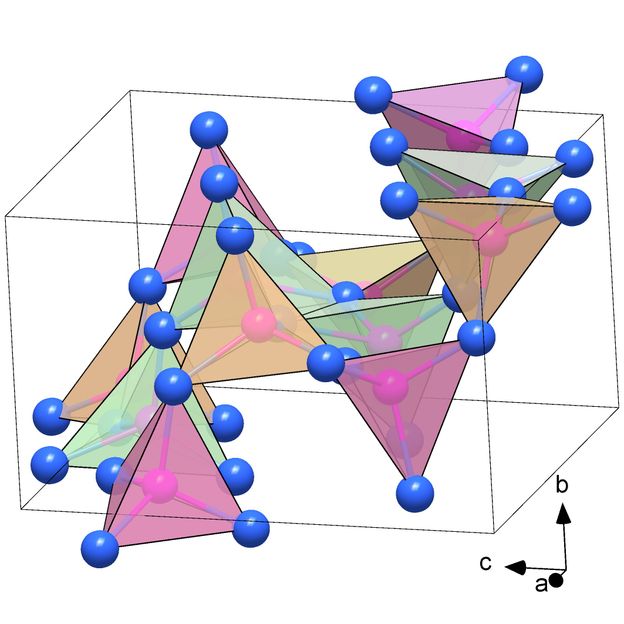
Likewise unusual: the structure, composed of PN₄ tetrahedra, of the polymorph α′-P₃N₅ which is a previously unknown modification of the polynitride P₃N₅.
(c) Dominique Laniel
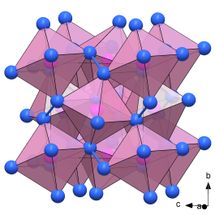
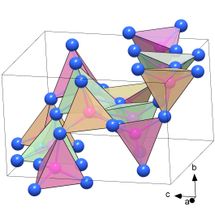
The researchers succeeded in synthesizing a previously unknown modification of the phosphorus nitride P₃N₅, the polymorph δ-P₃N₅, at a pressure of 72 gigapascals. At 134 gigapascals, the phosphorus nitride PN₂ formed in the diamond anvil cell. Both compounds are classified as ultra-incompressible materials with the bulk moduli above 320 GPa. The researchers discovered a key reason for this extreme strength through synchrotron X-ray diffraction analysis density functional theory calculations: crystal structures of δ-P₃N₅ and PN₂ consist of a dense network of PN₆ octahedra with a phosphorus atom surrounded by six nitrogen atoms. Until now, the existence of these structural units had only been suspected, but now they have been empirically proven for the first time.
The polymorph δ-P₃N₅ transformed into another, also previously unknown modification of P₃N₅ when the compression pressure was reduced: at seven gigapascals, the polymorph α′-P₃N₅ was formed. This is a new solid material that remains stable under normal ambient conditions. The crystal structure of this phosphoronitride is also unusual, being composed of PN₄ tetrahedra: A phosphorus atom is located in the center of these pyramid-shaped structural units, while the four "corners" are each occupied by a nitrogen atom. Compared with the well-known polymorph α-P₃N₅, which is already being discussed in research as a possible industrial material, α′-P₃N₅ has a significantly higher density. It is therefore considerably harder and potentially even more attractive in terms of potential engineering applications.
"The α′-P₃N₅ formed on decompression of δ-P₃N₅ exemplifies how nitrogen compounds with highly interesting properties can be discovered via a detour of high-pressure syntheses. Further investigations should now follow to explore potential applications of this new material. With our publication, we want to encourage more high-pressure and high-temperature research on non-metal nitrides – which have been largely neglected in comparison with metal nitrides. New studies in this exciting field can significantly expand our understanding of nitrogen chemistry. They will also potentially contribute to the discovery of recyclable materials for everyday products," says Bayreuth crystal physicist Prof. Dr. Dr. h.c. Natalia Dubrovinskaia from the Laboratory of Crystallography at the University of Bayreuth, who coordinated the research.
International cooperation
Together with the Bavarian Research Institute of Experimental Geochemistry & Geophysics (BGI) and the Laboratory of Crystallography at the University of Bayreuth, numerous other research partners were involved in the new study: the LMU Munich, the University of Edinburgh, the University of Linköping, Shandong University in Jinan, China, the German Electron Synchrotron (DESY) in Hamburg, the European Synchrotron Radiation Facility in Grenoble, and the Center for Advanced Radiation Sources at the University of Chicago.