Researchers tailor main-group catalyst with atomically dispersed in sites for highly efficient oxidative dehydrogenation
transition metal oxides are a kind of catalysts for oxidative dehydrogenation of alkanes. However, they suffer from inferior alkenes yield due to the trade-off between conversion and selectivity induced by more reactive alkenes than alkanes.
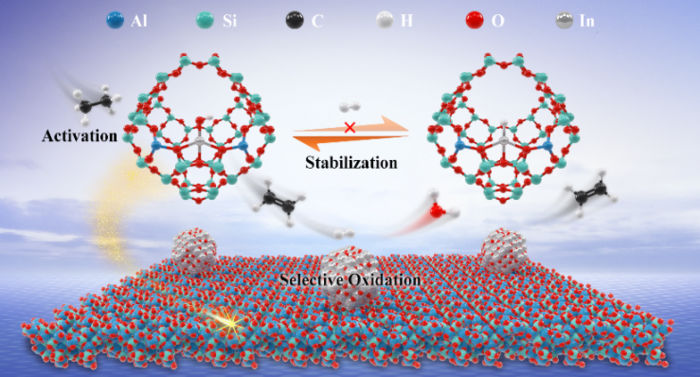
Schematic of the local coordination environment of isolated In site and proposed selective oxidative dehydrogenation process on main-group In catalyst
JACS
Recently, a research group led by Prof. WANG Xiaodong and Prof. ZHANG Tao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed and demonstrated a new concept to achieve high alkenes yields by regulating the activation of intrinsically selective catalyst for alkanes from weakness to strength.
This study was published in Journal of the American Chemical Society on Aug. 25.
The researchers designed a main-group catalyst with atomically dispersed In sites to disentangle the dilemma of trade-off between activity and selectivity in oxidative dehydrogenation process.
This novel catalyst exhibited exceeding 80% C2H4 selectivity at around 80% C2H4 conversion, thus achieving more than 60% C2H4 yield, which outperformed the state-of-the-art transition metal oxide catalysts.
Moreover, the researchers found that atomically dispersed [InOH]2+ sites anchored by substituting the protons of supercages in HY enabled the activation of ethane via significantly lowering the barrier of ethane dissociation and their structure could be stabilized by H2O formed from selective oxidation of hydrogen by In2O3 nanoparticles, thus exhibiting excellent performance for oxidative dehydrogenation of ethane.
"Our study unlocks new opportunities for the utilization of main-group elements and paves the way toward more rational design of catalysts for highly efficient selective oxidation catalysis," said Prof. WANG.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.




























































