Adhesion from Cold to Hot
Supramolecular adhesive with usable temperature range of 400 degrees Celsius
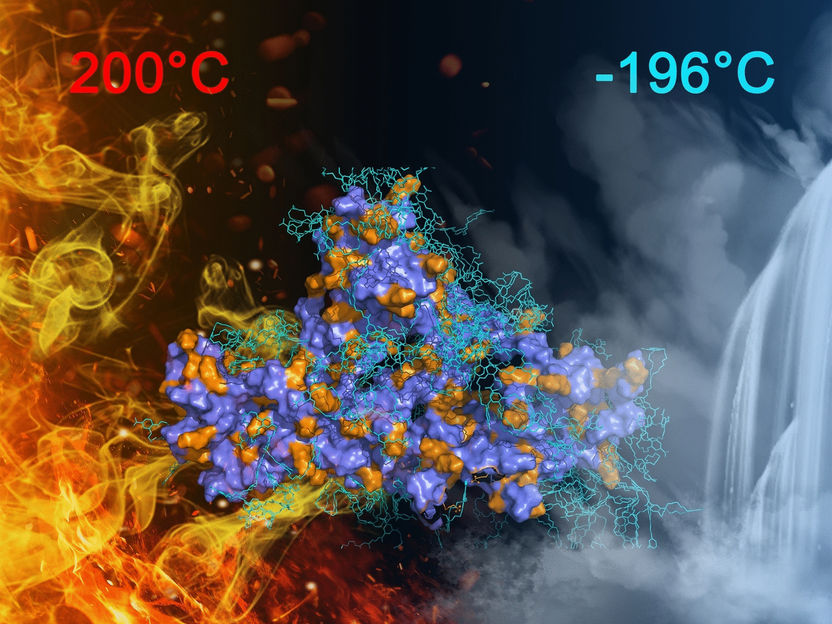
Researchers have developed a supramolecular adhesive that is recyclable and has outstanding gluing properties across a wide range of temperatures, from liquid nitrogen (−196 °C) up to oven-hot temperatures (plus 200 °C). As the team report in the journal Angewandte Chemie, the adhesive got its efficiency from an exceptionally tight interlocking of the molecular components during curing.
© Wiley-VCH
Unlike standard adhesives, supramolecular adhesives do not create adhesion by the molecular components crosslinking with one another. Instead, they form a tight-knit self-assembly during curing, like puzzle pieces fitting together. Researchers are interested in such supramolecular systems because they offer sustainability and customizability and, in principle, the individual starting materials can be recovered again and their chemical behavior can be tailored. However, to date, the performance of such glues has been decent at best, not to mention highly dependent on environmental conditions.
The new supramolecular glue, developed by a research team headed by Kai Liu from Tsinghua University, Beijing, China, consists of two components, one of which is a small protein that is synthesized in bacteria modified for the purpose. The other component is a crown ether—a ring-shaped molecule which can wrap snugly around another molecule, much like a crown sitting on a queen’s head.
The researchers observed this snug interaction between the molecules in their adhesive system. By adding the crown ether and the protein together and heating the solution for curing, the crown ether became anchored to the surface of the protein. The team noted that the protein and crown ether were so tightly bound to each other by their opposing charges and other molecular interactions that they formed a new, interlocking structure, which “welded” the proteins together.
The result was an extraordinarily strong adhesive effect. Steel plates glued together withstood high shear forces at room temperature, in liquid nitrogen, and at 200 °C. The adhesive worked for different materials, and under water as well. Such a broad spectrum of working conditions is seldom achieved, even with specialist adhesives, and is certainly a first for supramolecular adhesives. Promisingly, the interlocking components could be broken apart and recycled again, and the reused adhesive lost virtually none of its power.
The researchers believe that one reason for this exceptional adhesive effect, particularly at low temperatures, is a result of the specific supramolecular interactions at play. In particular, the tight interlocking of the components drove water out of the protein. This meant that no ice crystals were able to form when frozen—as in antifreeze—which in many conventional glues would lead to premature cracking.
The researchers suggest that this new adhesive could be applied to the manufacture of special parts that will be subject to greatly fluctuating conditions during use; for example, the wide temperature ranges to which spacecraft are exposed.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.