In control of chaos: On the hunt for high entropy crystals
Special synthesis device can test many different chemical mixtures one after the other, as if on an assembly line
Crystals consisting of wildly mixed ingredients - so-called high-entropy materials - are currently attracting growing scientific interest. Their advantage is that they are particularly stable at extremely high temperatures and could be used, for example, for energy storage and chemical production processes. An Empa team is producing and researching these mysterious ceramic materials, which have only been known since 2015.
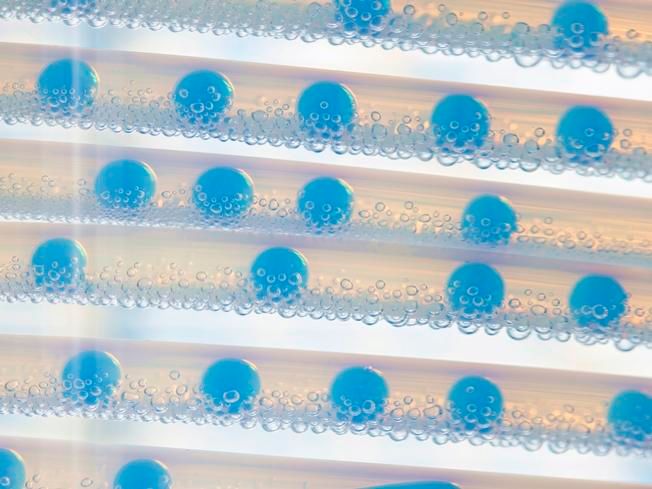
Assembly line: A different chemical mixture is created in each of the droplets within the "Tubular flow reactor" – under exactly the same boundary conditions.
Empa
Nature strives for chaos. That's a nice, comforting phrase when yet another coffee cup has toppled over the computer keyboard and you imagine you could wish the sugary, milky brew back into the coffee cup - where it had been just seconds before. But wishing won't work. Because, as mentioned, nature strives for chaos.
Scientists have coined the term entropy for this effect - a measure of disorder. In most cases, if the disorder increases, processes run spontaneously and the way back to the previously prevailing order is blocked. See the spilled coffee cup. Even thermal power plants, which generate a huge cloud of steam above their cooling tower from a neat pile of wood or a heap of hard coal, operate driven by entropy. Disorder increases dramatically in many combustion processes - and humans take advantage of this, tapping a bit of energy in the form of electricity from the ongoing process for their own purposes.
Can entropy stabilize anything?
Crystals are considered the sheer opposite of disorder. In a crystal structure, all the elements of the lattice are neatly sorted close together in the smallest possible volume. This makes the idea that crystals can be stabilized by the force of entropy and thus create a new class of materials all the more bizarre.
Entropy-stabilized materials are still a young field of research. It began in 2004 with so-called high-entropy alloys, i.e. mixtures of five or more elements that can be mixed together. If the mixture is successful and all the elements are homogeneously distributed, special properties sometimes emerge that do not come from the individual ingredients but from their mixture. Scientists call these "cocktail effects".
Even in the heat, the chaos reigns
Since 2015, it has been known that even ceramic crystals can be stabilized by the "power of disorder." In this way, even oversized and miniscule elements fit into the crystal, which would normally destroy it. The Empa research team has already succeeded in inserting nine different atoms into a crystal. The advantage is that they remain stable even at high temperatures - because "rearranging" them would lead to greater order. The natural striving for maximum disorder thus stabilizes the unusual crystal structure - and thus the entire material.
"With up to four components in the crystal, everything is still normal; with five components and more, the world changes," explains Michael Stuer, a researcher in Empa's High Performance Ceramics department. Since the Luxembourg-raised researcher joined Empa in 2019, he has been working on the research field of high-entropy crystals. "This class of materials opens up a wide range of new opportunities for us," Stuer says. "We can stabilize crystals that would otherwise disintegrate due to internal stresses. And we can create highly active crystal surfaces that have never existed before and look for interesting cocktail effects."
Together with his colleague Amy Knorpp, Stuer is now setting out into the unknown. The two are specialists in the production of fine crystal powder, and they have colleagues at Empa for X-ray and surface analysis to precisely characterize the samples they produce. With their help, Michael Stuer now wants to be at the forefront of the international scene. "The number of publications on the subject of high entropy crystals is increasing very strongly right now. And we want to be there right from the start," says the researcher.
Islands of knowledge
What is needed now is a systematic approach, expertise and a good dose of perseverance. Where do you start? What direction does one take? "At the moment, there is no coherent expertise, no complete overview of this new field of research," Stuer says. "Different research groups around the world are working on limited projects. So individual islands of knowledge are emerging that will have to grow together over the next few years."
Michel Stuer and Amy Knorpp focus on catalytically active materials. The chemical reaction they are interested in involves combining CO2 and hydrogen to form methane. The aim is to turn a greenhouse gas into a sustainable, storable fuel. "We know that CO2 molecules adsorb particularly well on certain surfaces and that the desired reaction then takes place more easily and quickly," says Amy Knorpp. "Now we are trying to produce entropic crystals on whose surfaces such highly active regions exist."
Chemical assembly line
To make progress faster, the researchers have built a special synthesis device with the help of Empa's workshop, in which many different chemical mixtures can be tested one after the other, as if on an assembly line. In the "Segmented Flow Tubular Reactor", small bubbles run through a tube in which the respective reaction takes place. At the end, the bubbles are emptied and the powder they contain can be further processed.
"The 'Tubular Flow Reactor' has a huge advantage for us: all the bubbles are the same size, which is why we always have ideal and consistent boundary conditions for our syntheses," explains Stuer. "If we need larger quantities of a particularly promising mixture, we simply produce several bubbles with the same mixture one after the other."
The windows on the right side
The precursor powder is then turned into fine crystals of the desired size and shape through various drying processes. "Crystals are like houses, they have closed outer walls and some with windows," explains Michael Stuer. Sometimes the shape of the crystal already indicates the window side. For example, when a mixture forms needle-shaped crystals. "The long sides of the needle are the lower-energy ones. Not much happens there. The crystal edges at the tips of the needles, on the other hand, are high-energy. That's where it gets interesting," Stuer said.
For their first major project, the Empa researchers have teamed up with colleagues from the Paul Scherrer Institute (PSI). They are investigating the possible methanation of CO2 from biogas plants and sewage treatment plants in an experimental reactor. The PSI researchers have already gained experience with various catalysts and repeatedly encounter a problem: The catalyst, on whose surface the chemical reaction takes place, weakens over time. This is due to the fact that sulfur components in the biogas contaminate the surface or that the catalyst surfaces undergo chemical transformation at high temperatures.
This is where the researchers are looking for a breakthrough using entropic crystals; after all, these do not break down even at high temperatures - they are stabilized by chaos. "We're holding out hope that our crystals will last longer in the process and possibly be more impervious to sulfur pollution," Stuer says.
Drawing a map
After that, Empa's crystal specialists are ready for other challenges, such as high-performance batteries, superconducting ceramics or catalysts for car exhaust and other chemical production processes. "It's a dark forest we're walking into," says Amy Knorpp. "But we have a guess in which direction something might be found. Now we're drawing a map of these systems. Somewhere out there, we think, is a treasure chest of insights hidden away."