Novel method examines the gas-liquid interface in new detail
Novel molecular beam scattering apparatus that uses a liquid flat jet can study chemical reactions at the gas liquid interface of volatile liquids
The interface between gases and liquids is found throughout nature. It is also important to many industrial processes. To improve understanding of the gas-liquid interface, researchers have developed an apparatus to study reactions between gas molecules and highly volatile liquids with new levels of detail. It uses a molecular beam that is directed onto a flat liquid surface. When the beam scatters, a detector collects data on the speed, direction, and mass of molecules in the scattered beam. This allows researchers to deduce the changes related to the interaction of gas and liquid. To evaluate the feasibility of this novel approach, the researchers studied the interaction between the noble gas neon and liquid dodecane.
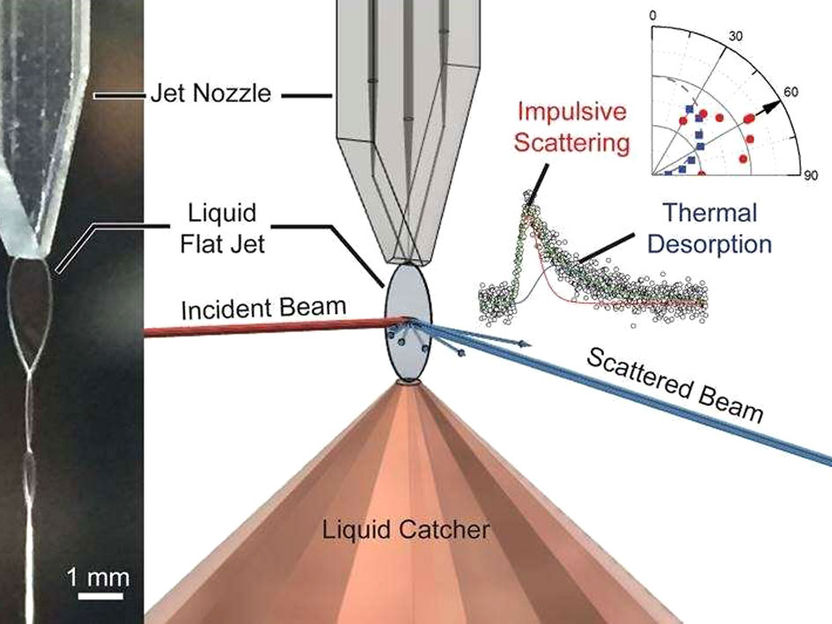
Left: a liquid dodecane flat jet produced by a microfluidic chip nozzle. Right: an incident molecular beam (red line) striking the jet surface. Researchers can analyze the velocity and angular distributions of molecules in the scattered beam (blue line).
Image courtesy of Chin Lee, University of California at Berkeley
The Impact
The interface between the gas and liquid phase is a unique chemical environment. It is important to understand chemical reactions in the Earth’s atmosphere and how carbon moves between the air and the surface of the sea. In industrial settings, this interface affects how air and fuel mix in internal combustion engines and other applications. The novel flat jet scattering apparatus opens new opportunities for gas‑liquid interface studies of volatile liquids. Scientists can now study reactions of molecules on the liquid water surface with molecular-level resolution. The researchers plan to use this method to study the formation of acid rain and molecules related to air pollution.
Summary
This research reports the first results of a newly designed flat jet scattering apparatus. The researchers, including scientists from the University of California, Berkeley; Lawrence Berkeley National Laboratory; the Fritz Haber Institute of the Max Planck Society; the Leibniz Institute of Surface Engineering; and the University of Leipzig, demonstrated the feasibility of the apparatus by studying the neon‑liquid dodecane scattering system. They started by measuring molecular evaporation from a neon‑doped dodecane flat jet. The research found that evaporation follows an angular distribution that is best approximated by a cosine function for both neon and dodecane molecules. Also, the velocity distribution of the outgoing neon molecules follows a Maxwell‑Boltzmann distribution at the liquid temperature. This indicates unperturbed evaporation of neon. The researchers therefore used neon atoms to probe the scattering dynamics at the liquid dodecane surface.
In the scattering experiments, the team observed two main mechanisms: impulsive scattering (IS) and thermal desorption (TD). In TD, molecules impinging on the surface fully thermalize with the liquid and subsequently desorb. This mechanism has a fingerprint already known from the evaporation studies. For IS, however, information about the initial beam energy and direction is partially conserved. The research exploited this condition to quantify the translational energy transfer from neon to the liquid. They showed that the nature of the energy transfer can be modeled with a soft‑sphere kinematic model. This model enabled them to estimate the effective surface mass of dodecane to be 60 amu, which is much smaller than a single dodecane molecule (170 amu), thereby indicating that only part of a dodecane molecule contributes to the interaction at the collision timescale. The team’s next steps include conducting experiments related to protic/aprotic molecular scattering off dodecane and reactive scattering from water.
Funding:
This work is funded by the Department of Energy (DOE) Office of Science, Basic Energy Science (BES) Program, Chemical Sciences, Geosciences, and Biosciences Division. This research used the resources of the SLAC National Accelerator Laboratory, also supported by the DOE Office of Science, BES Program. The Alexander von Humboldt Foundation and the German Research Foundation provided additional support.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
American_Society_of_Echocardiography

When heat turns into electricity at 1000 °C - New perspectives for producing electricity from industrial waste heat
List_of_drugs:_Df-Di
Microscopic Octopuses from a 3D Printer - Newly developed smart polymers have “life-like” properties

GRYF HB spol. s.r.o - Havlíčkův Brod, Czechia
Artificial intelligence for obtaining chemical fingerprints - Neural networks carry out chemical simulations in record time



























































