Scientists serendipitously discover rare cluster compound
The molecule is unusual and has ‘great potential’ in catalysis, conduction and other applications
Scientists at Kyoto University’s Institute for Cell-Material Sciences have discovered a novel cluster compound that could prove useful as a catalyst. Compounds, called polyoxometalates, contain a large metal-oxide cluster carry a negative charge. They are found everywhere, from anti-viral medicines to rechargeable batteries and flash memory devices.
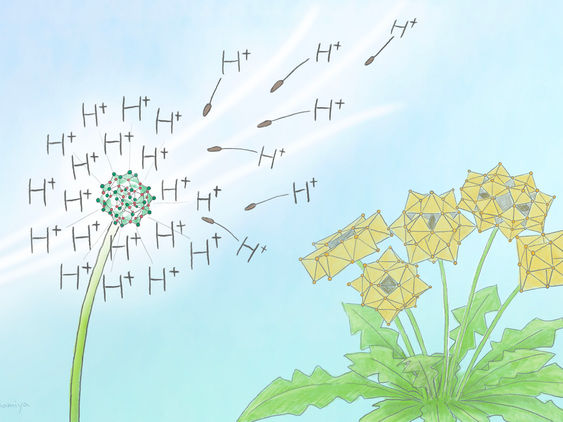
The metal oxide clusters obtained in this study are positively (+) charged unlike conventional negatively (-) charged ones. Its surface protons are highly acidic, which is important in catalysis.
Mindy Takamiya/Kyoto University iCeMS
The new cluster compound is a hydroxy-iodide (HSbOI) and is unusual, as it has large, positively charged clusters. Only a handful of such positively charged cluster compounds have been found and studied.
“In science, the discovery of new material or molecule can create a new science,” says Kyoto University chemist Hiroshi Kageyama. “I believe that these new positively charged clusters have great potential.”
The first metal oxide cluster was discovered in 1826. Chemists have since synthesised hundreds of compounds with negatively charged clusters, which have properties useful in magnetism, catalysis, ionic conduction, biological applications and quantum information. Their properties make them useful in diverse fields from catalysis to medicine and chemical synthesis.
In more recent years, scientists have focused their attention on synthesising compounds with positively charged clusters and learning their properties.
Kageyama and his colleague Ryu Abe found their positive cluster by accident. Since 2016, the two scientists – Kageyama, a solid-state chemist and Abe, a catalytic chemist – have been on a quest to develop new compounds that can absorb visible light for photocatalysis. They were studying a chlorine-containing (Sb4O5Cl2) compound and trying to replace the chlorine atom with iodine.
“However, a new material that was completely different from what we expected was obtained accidentally,” says Kageyama.
What the scientists expected was a material that contains 22 atoms in the unit cell. What they got instead was a compound that contains 800 atoms in its unit cell.
At the beginning, the scientists could not unravel the chemical’s structure. A traditional technique called powder X-ray diffraction failed when faced with the material’s complexity. After a year, Kageyama thought he could use three-dimensional electron tomography, a cutting-edge electron microscopy technique that has attracted recent attention as a tool to image the structure of proteins. The scientists approached Artem Abakumov and Joke Hadermann at University of Antwerp, Belgium, to work on the structure. And when their collaborators sent the data back, the scientists were thrilled to see large clusters.
Further lab work showed the hydroxyiodide molecule contained acidic protons, which is important in catalysis.
“This finding may open up new possibilities in the design of solid-state catalysts,” says Kageyama.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.