Catalyst from hot water
Environmentally friendly hydrothermal synthesis of a substance with both organic and inorganic properties in a single process
Advertisement
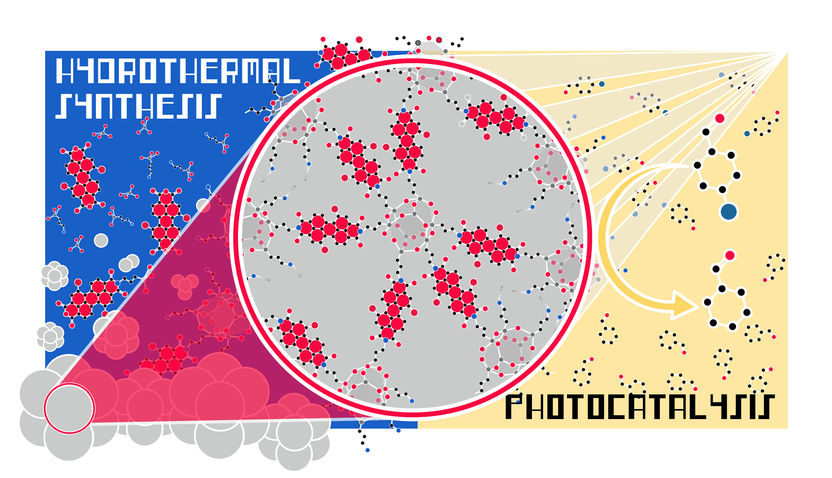
The production of chemical substances normally requires environmentally harmful solvents. After the research group of Miriam Unterlass, professor of solid state chemistry at the University of Konstanz, produced organic substances without harmful substances for the first time by heating them in hot water, the researchers can now chalk up another success: Through hydrothermal synthesis, they succeeded in jointly forming and combining organic and inorganic substances in the same reaction vessel. Specifically: an inorganic solid that encloses organic dye molecules. When exposed to light, which is by and large the most environmentally friendly energy resource, the hybrid material functions like a catalyst, i.e., a photocatalyst. Since the photocatalyst is a solid, it can be used multiple times.
D. Alonso Cerrón-Infantes
The study was recently published online by the Journal of Materials Chemistry A. In the following printed edition of the journal (issue 24, year 2022) the study will be featured on the cover page, which is reflecting a special appreciation.
Hydrothermal synthesis, i.e. the production of materials under pressure in hot water, is copied from nature. In underground hot water reservoirs, for example, rock crystals form as the atoms dissolved in the hot water react with each other, first forming molecules and then crystals. In the same way, inorganic molecules can be produced in synthetic chemistry – and as described in a study on the environmentally friendly process in the synthesis of organic substances from 2021 by Miriam Unterlass – also organic molecules without toxic solvents.
Environmentally friendly synergy of both processes
An environmentally friendly synergy of both methods arises from the current results, in which first author Dr Hipassia Moura, a postdoctoral researcher in Miriam Unterlass' team, plays a major role. Miriam Unterlass: "In our work we show that it is possible to form inorganic and organic substances at the same time in ‘hot water’, and that something useful comes out of it."
The fact that the hybrid material can be produced completely without toxic solvents is all the more remarkable because the chemist's research team works with dye molecules that normally require highly toxic chemicals for their synthesis. The core of the new substance, which was created in hot water, is formed by dye molecules that exist as a solution, while the material surrounding them has the properties of a solid. The result is a solid that behaves like a solution in terms of optical properties.
Reusable catalyst
Dyes as solutions have very specific properties. The dye molecules used by Miriam Unterlass' research team are able to absorb light and thus catalyze reactions. This process is similar to photosynthesis in plants, where it is also pigments that absorb the light needed for photosynthesis. Unlike a solution that has to be disposed of after use, the hybrid material has the added advantage that it can be used again and again as a catalyst, because it is like a solid on the outside.
The research team's specific target for application are small organic molecules that play a role in pharmaceuticals. In principle, however, the method is relevant for various chemical reactions and thus the production of countless synthetic products. And while water still has to be heated for the synthesis of the hybrid material, only light energy is required for the catalytic effect. "Light is the best resource we have. Light cannot be used up", says Miriam Unterlass.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.































































