Hybrid electro-biosystem upcycles carbon dioxide into energy-rich long-chain compounds
Upcycling CO₂ via electrochemical and metabolic engineering
Artificial upcycling of carbon dioxide (CO2) into value-added products in a sustainable manner represents an opportunity to tackle environmental issues and realize a circular economy.
The conversion of carbon dioxide and water into long-chain products realized by electrochemically coupled biological fermentation
SIAT
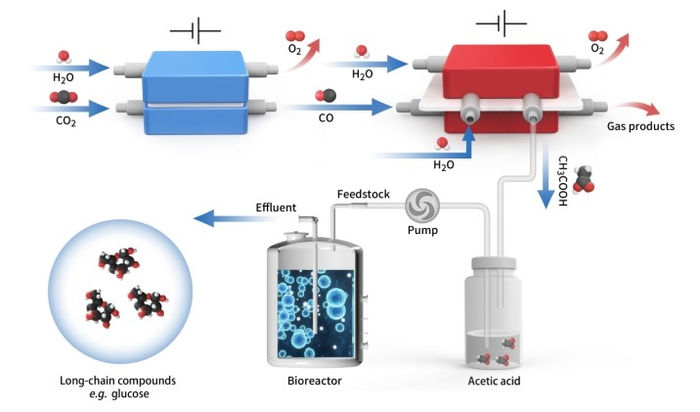
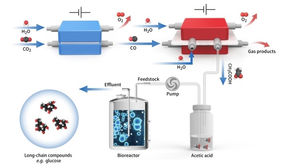
Schematic diagram of in vitro carbon dioxide synthesis of high energy long chain food molecules
SIAT

However, compared with facilely available C1/C2 products, efficient and sustainable synthesis of energy-rich long-chain compounds from CO2 still remains a huge challenge.
A joint research team led by Prof. XIA Chuan from the University of Electronic Science and Technology of China, Prof. YU Tao from the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences, and Prof. ZENG Jie from the University of Science and Technology of China, has developed a hybrid electro-biosystem, coupling spatially separate CO2 electrolysis with yeast fermentation, which efficiently converted CO2 to glucose.
The proposed spatially decoupled electro-biosystem includes CO2 electrolysis and yeast fermentation. It can convert CO2 to glucose or fatty acids with both high titer and high yield.
"Acetic acid is not only the main component of vinegar, but also one of the excellent biosynthetic carbon sources. It can be transformed into other substances in life, such as glucose. Acetic acid can be obtained by direct electrolysis of CO2, but with ultra-low efficiency. We thus propose a two-step strategy to convert CO2 into acetic acid, with CO as the intermediate," said Prof. ZENG.
Accordingly, the researchers first converted CO2 into CO in a membrane electrode assembly using a Ni–N–C single-atom catalyst, and then developed a grain-boundary-rich Cu (GB_Cu) catalyst for acetate production from electrochemical CO reduction.
GB_Cu exhibited a high acetate Faradaic efficiency up to 52% at -0.67 V versus a reversible hydrogen electrode in a typical three-electrode flow cell reactor using 1.0 M KOH aqueous electrolyte.
"However, the acetate produced by conventional electrocatalytic devices is always mixed with electrolyte salts which cannot be directly used for biological fermentation," said Prof. XIA.
To tackle this challenge, the researchers developed a porous solid electrolyte reactor equipment with thick anion exchange membranes for pure acetic acid solution separation and purification. It continuously and stably worked for 140 hours under a current density of -250 mA cm-2, which achieved an ultrapure acetic acid solution with a relative purity of ~97% wt.%.
In the following microbial fermentation, the researchers deleted all defined hexokinase genes (glk1, hxk1, hxk2, YLR446W and emi2) in Saccharomyces cerevisiae to enable microbe growth on pure acetic acid and the efficient release of glucose in vitro.
The overexpression of heterologous glucose-1-phosphatase further improved the glucose titer. S. cerevisiae was fed with titrated acetate from electrolysis, obtaining an average glucose titer of 1.81 ± 0.14 g·L-1, equivalent to a high yield of 8.9 μmol per gram of yeast per hour. Similar results were observed in S. cerevisiae fed pure acetic acid.
In addition, an engineered S. cerevisiae for free fatty acids production was fed via titrating acetate from electrolysis, with a total free fatty acids (C8~C18) titer of 500 mg·L-1.
Pure and concentrated acetic acid from electrochemical CO2 reduction served as the carbon source for S. cerevisiae fermentation. Such a platform for long-chain products is promising for large-scale practical use.
"This demonstration is a starting point for realizing light-reaction-free artificial synthesis of important organic products from CO2," said Prof. YU.






























































