New materials for storing flammable industrial gases
Advertisement
How do I store more, and better? This summarizes the challenge of transporting flammable gases. To ensure industrial safety, these gases must be handled at defined temperature and pressure conditions that do not allow for optimal storage and release cycles. Existing porous materials can facilitate the capture of certain gases, but their high affinity for these molecules complicates their release: a large amount of gas then remains trapped in the host material.
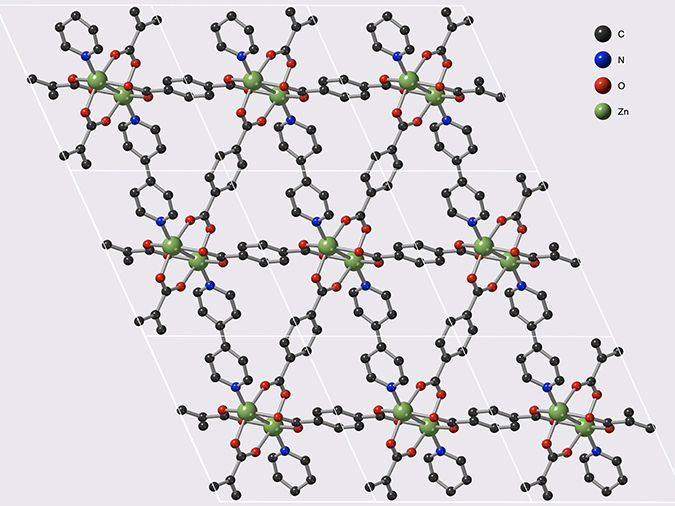
Structure of the Metal-Organic Framework MOF-508, composed of carbon (black), nitrogen (blue), oxygen (red) and zinc (green). The flexibility and catenated nature of this framework are key parameters for the storage of acetylene.
© François-Xavier Coudert/CNRS
Scientists have just shown that new patented materials could provide a solution, by demonstrating their ability to capture and release acetylene. For a given volume, they can store and release 90 times more acetylene. In that step, it is even possible to recover 77% of the gas stored in a cylinder – far more than with existing porous materials. And all this is at temperature and pressure conditions suitable for industrial applications.
These materials belong to the family of Metal-Organic frameworks (MOFs) that form nanoporous crystal structures. The MOFs studied during this work have the peculiarity of being flexible, and thus offer two states: "open" and "closed", facilitating gas storage and release respectively. In addition, they can be modified to control the storage–release pressure very finely, and thus be suitable for various industrial constraints.
Based on these results, the research team plans to test new modifications to give these flexible MOFs novel properties, for example to facilitate the capture of CO2, methane or hydrogen. Reducing the cost of these new materials remains a major objective in order to develop industrial applications.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.