Ethers may have a more sustainable future thanks to heterogenous catalysis
Researchers report a zirconium oxide-supported platinum-molybdenum catalyst for the preparation of unsymmetrical ethers from esters
Advertisement
Optimizing chemical processes to ensure they are environmentally friendly and sustainable is becoming increasingly important and catalysts play a key role as they can make reactions more efficient. Researchers from Osaka University have reported a zirconium oxide-supported platinum-molybdenum catalyst that promotes the selective conversion of esters into valuable unsymmetrical ethers. Their findings are published in JACS Au.
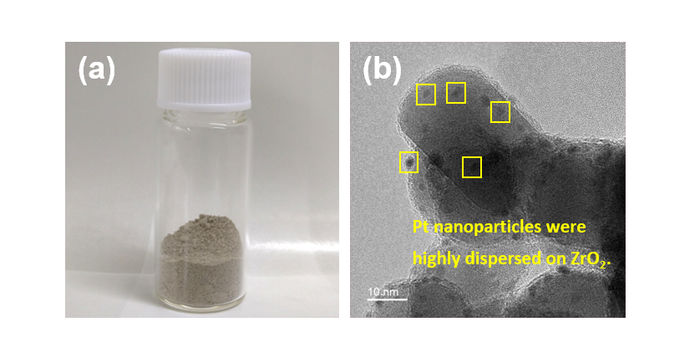
(a) The photo of Pt‒Mo/ZrO2 catalyst. (b) Transmission electron microscope image of Pt‒Mo/ZrO2.
Katsumasa Sakoda et al.
Catalysts have long been at the heart of making chemical reactions faster, improving their yields, and making them less wasteful and more energy efficient, leading to processes that are more cost effective and better for the environment.
Ethers are organic molecules that have a range of uses in products such as fragrances, fuels, and pharmaceuticals. They contain an oxygen atom that can be thought of as a bridge between two parts. If the parts are the same, they are considered symmetrical; if they are different, they are unsymmetrical. Current routes to unsymmetrical ethers have various limitations. For example, some require specific starting materials, some produce large amounts of waste, and some need conditions—such as high pressure—that are expensive and/or polluting to produce.
Using just hydrogen to convert esters into ethers through direct hydrodeoxygenation can be thought of a hydrogen molecule (H2) grabbing an oxygen atom out of the ester leaving the ether and a water molecule (H2O). This is both efficient and clean; therefore, developing catalysts to promote this reaction under mild conditions has significant advantages.
“Our zirconium oxide-supported platinum-molybdenum catalyst allowed us to obtain good yields of over 20 unsymmetrical ethers under mild conditions using hydrogen molecules,” explains co-author of the study Sho Yamaguchi. “This is very encouraging because there are numerous naturally occurring and low-cost esters that can be made into more valuable ether products.”
The catalyst was easily separated from the reaction mixtures and could be reused without losing activity. It was also found to work when the hydrogen was at atmospheric pressure. In addition, the researchers showed that it was possible to convert a specific triglyceride derived from biomass into the corresponding triether. Biomass is a renewable resource, therefore processes appropriate for biomass-derived materials provide sustainable solutions.
“Our catalyst has significant potential for the environmentally friendly and sustainable production of unsymmetrical ethers,” says study corresponding author Tomoo Mizugaki. “If we are to continue enjoying the variety of products available today and maintain development at the rate we are accustomed to, catalysts such as ours will be key to an efficient and clean future.”

































































