"Magic" combination for more effective hydrogenations
The activity of this “magic” combination came as a real surprise
hydrogen (H2) is the smallest chemical molecule and a beacon of hope for a more environmentally friendly energy transition in the coming years. In addition, it is already used in a variety of industrial processes - so-called hydrogenations - for the environmentally friendly production of chemical products. For hydrogen to be used both for energy production and in hydrogenations, it is necessary to selectively activate the relatively stable hydrogen-hydrogen bond and allow it to react further. These molecular processes require catalysts, which are usually based on expensive and comparatively rare precious metals such as platinum, palladium or rhodium.
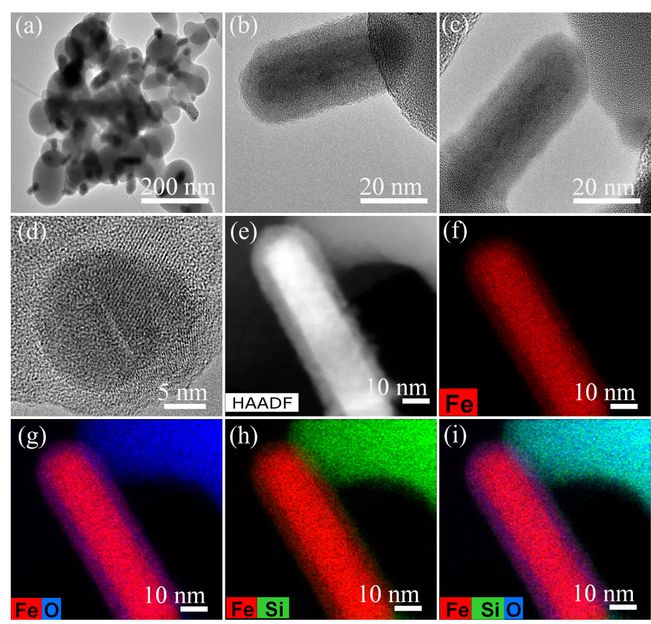
TEM and HRTEM images of Fe/Fe-O@SiO2 catalyst. Upper row: distribution of Fe-nanoparticles. Middle: Fe/FeO core-shell structured nanoparticle. Bottom: Elemental [iron(Fe), silica (Si) and oxygen (O)] mapping.
Vishwas Chandrashekhar, LIKAT
In a research collaboration between the Leibniz Institute for Catalysis (LIKAT) in Rostock, Germany and the Regional Centre of Advanced Technologies and Materials (RCPTM) at Palacký University Olomouc in the Czech Republic, it has now been possible to develop significantly simpler catalyst materials based on inexpensive iron and silicon dioxide (sand), which can be used to carry out various hydrogenation reactions, for the production of intermediates for pesticides, active pharmaceutical ingredients as well as plastics, much more efficiently.
To this end, Vishwas Chandrashekhar, a doctoral student in the group of Prof. Matthias Beller and Prof. Jagadeesh Rajenahally at LIKAT, has produced a large number of nanostructured iron-based catalysts that only develop their actual activity in the presence of cheap aluminum compounds. The activity of this “magic” combination came as a real surprise. Czech scientists led by Prof. Radek Zboril at the renowned RCPTM subsequently succeeded in systematically characterizing the novel materials using state-of-the-art analytical techniques.
The optimal iron catalyst with the technical designation Fe/Fe-O@SiO2 is a well-defined nanostructured material that has a so-called fayalite structure at the interface between silicon dioxide and iron. Fayalite is a rare, naturally occurring iron silicate mineral. Another special feature of the developed catalyst is α-Fe nanoparticles at this interface. These nanoparticles are surrounded by an ultrathin amorphous iron(III) oxide layer, in other words "rust", which virtually grows out of the silica structure.
The scientists from Rostock and Olomouc believe that their work will have a significant impact on global efforts to find low-cost hydrogenation catalysts, and that it will open new doors for using iron-based nanostructured catalysts in other challenging hydrogenations.
Original publication
Vishwas G. Chandrashekhar, Thirusangumurugan Senthamarai, Ravishankar G. Kadam, Ondřej Malina, Josef Kašlík, Radek Zbořil, Manoj B. Gawande, Rajenahally V. Jagadeesh & Matthias Beller; "Silica-supported Fe/Fe–O nanoparticles for the catalytic hydrogenation of nitriles to amines in the presence of aluminium additives"; Nature Catalysis, 2022
Most read news
Original publication
Vishwas G. Chandrashekhar, Thirusangumurugan Senthamarai, Ravishankar G. Kadam, Ondřej Malina, Josef Kašlík, Radek Zbořil, Manoj B. Gawande, Rajenahally V. Jagadeesh & Matthias Beller; "Silica-supported Fe/Fe–O nanoparticles for the catalytic hydrogenation of nitriles to amines in the presence of aluminium additives"; Nature Catalysis, 2022
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Haplogroup_N_(Y-DNA)
Glycoside
Lots of lead in the water? Maybe manganese is to blame - Researchers have found the naturally occurring mineral can speed up the production of lead dioxide under certain circumstances





























































