First programmable photocatalyst developed
With smart materials toward more sustainable chemistry
Advertisement
Researchers at the Max Planck Institute of Colloids and Interfaces have developed a sustainable and "smart photocatalyst". The special feature: as a so-called smart material, it can distinguish between the colors of light (blue, red and green) and, in response, enables a specific chemical reaction programmed into it. "Our smart photocatalyst functions as a traffic guide who opens one specific pathway in response to light of specific color," says Dr. Yevheniia Markushyna, first author of the paper.
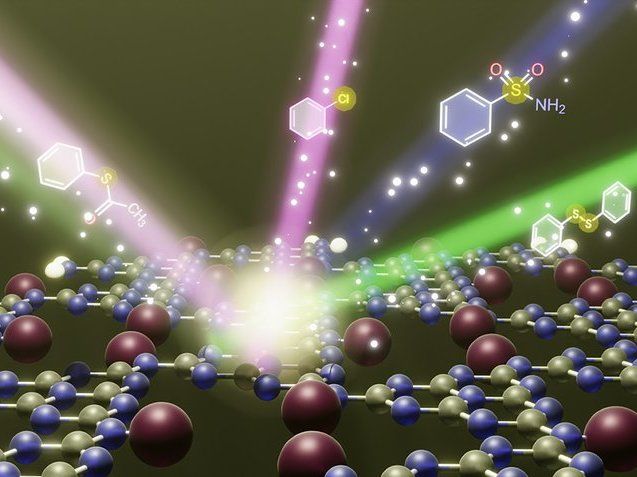
Atomic structure of carbon nitride photocatalyst represented by spheres of different colors (the atoms). A molecule of S-acetylthiophenol (on the left) approaches the carbon nitride photocatalyst. When light of specific color (green, blue or violet) hits the surface, the carbon nitride photocatalyst converts the molecule of S-acetylthiophenol into diphenyldisulfide (highlighted with green beam), sulfonylamide (highlighted with blue light) or chlorobenzene (highlighted with violet light).
Max-Planck-Institut für Kolloid- und Grenzflächenforschung / Aleksandr Savateev
Photocatalysts are special materials that use the energy from sunlight or LED light to enable a desired reaction. Often, this results in not just one product, but a variety. Chemists call this "poor selectivity" because separation of the desired product from the mixture consumes time and resources.
Quite different with the new method developed at the Max Planck Institute, which enables the research team for instance to synthesize sulfonamides in a targeted manner. Sulfonamides are organosulfur compounds that are used, among other things, as antibiotics to treat bacterial infections. The researchers have created a photocatalytically active carbon nitride material that produces with high selectivity sulfonamides. With the help of the sustainable "smart photocatalyst," one product is created selectively from three possible from the same reagent by adjusting the color of the incident light. "The special feature is that we can control the selectivity of the chemical reaction by turning on the light bulb of the right color," says Dr. Yevheniia Markushyna. "Today, we have sustainable smart photocatalysts and the knowledge to produce value-added organic compounds using solar light in the most efficient way possible," says Dr. Aleksandr Savateev, group leader and head of the photocatalysis study recently published in the journal Angewandte Chemie. He adds, "Potentially, our method could also make the production of sulfonamide antibiotics more sustainable."
Function
Complex biological objects, such as the human eye or state-of-the-art cameras in electronic devices can perceive light colors. It is a great challenge to develop "smart molecules" consisting of only tens of atoms. Such molecule must not only recognize the light colors (blue, red and green), but also perform a certain “programmed” action that depends on the specific light color.


























































