Decoding electron dynamics
A new method for identifying quantum orbits enables photoelectron spectroscopy via tunneling ionization to provide attosecond temporal and subangstrom spatial resolution measurement of electron dynamics
Electron motion in atoms and molecules is of fundamental importance to many physical, biological, and chemical processes. Exploring electron dynamics within atoms and molecules is essential for understanding and manipulating these phenomena. Pump-probe spectroscopy is the conventional technique. The 1999 Nobel Prize in Chemistry provides a well-known example wherein femtosecond pumped laser pulses served to probe the atomic motion involved in chemical reactions. However, because the timescale of electron motion within atoms and molecules is on the order of attoseconds (10-18 seconds) rather than femtoseconds (10-15 seconds), attosecond pulses are required to probe electron motion. With the development of the attosecond technology, lasers with pulse durations shorter than 100 attoseconds have become available, providing opportunities for probing and manipulating electron dynamics in atoms and molecules.
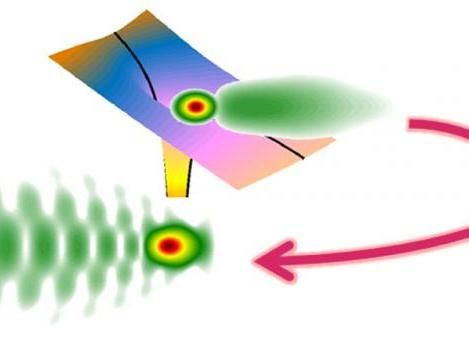
Hologram generated by the multi-orbit contribution from strong-field tunneling ionization.
Y. Zhang
Another important method for probing electron dynamics is based on strong-field tunneling ionization. In this method, a strong femtosecond laser is employed to induce tunneling ionization, a quantum mechanical phenomenon that causes electrons to tunnel through the potential barrier and escape from the atom or molecule. This process provides photoelectron-encoded information about ultrafast electron dynamics. Based on the relationship between the ionization time and the final momentum of the tunneling ionized photoelectron, electron dynamics can be observed with attosecond-scale resolution.
The relationship between ionization time and the final momentum of the tunneling photoelectron has been theoretically established in terms of a "quantum orbit" model and the accuracy of the relationship has been verified experimentally. But which quantum orbits contribute to the photoelectron yield in strong-field tunneling ionization has remained a mystery, as well as how different orbits correspond differently to momentum and ionization times. So, identifying the quantum orbits is vital to the study of ultrafast dynamic processes using tunneling ionization.
As reported in Advanced Photonics, researchers at Huazhong University of Science and Technology (HUST) proposed a scheme to identify and weigh the quantum orbits in strong-field tunneling ionization. In their scheme, a second harmonic (SH) frequency is introduced to perturb the tunneling ionization process. The perturbation SH is much weaker than the fundamental field, so it does not change the final momentum of the electron that is tunneling toward ionization. However, it can significantly alter the photoelectron yield, due to the highly nonlinear nature of tunneling ionization. Because of different ionization times, different quantum orbitals have different responses to the intervening SH field. By changing the phase of the SH field relative to the fundamental driving field and monitoring the responses of the photoelectron yield, the quantum orbits of tunneling ionized electrons can be accurately identified. Based on this scheme, the mysteries of the so-called "long" and "short" quantum orbits in strong-field tunneling ionization can be resolved, and their relative contribution to the photoelectron yield at each momentum is able to be accurately weighted. This is a very important development for the application of strong-field tunneling ionization as a method of photoelectron spectroscopy.
A collaborative team effort led by HUST graduate students Jia Tan, under the supervision of Professor Yueming Zhou, along with Shengliang Xu and Xu Han, under the supervision of Professor Qingbin Zhang, the study indicates that the hologram generated by the multi-orbit contribution from the photoelectronic spectrum can provide valuable information regarding the phase of the tunneled electron. Its wave packet encodes rich information about atomic and molecular electron dynamics. According to Peixiang Lu, HUST professor, vice director of the Wuhan National Laboratory for Optoelectronics, and senior author of the study, "Attosecond temporal and subangstrom spatial resolution measurement of electron dynamics is made possible by this new scheme for resolving and weighing quantum orbits."
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!