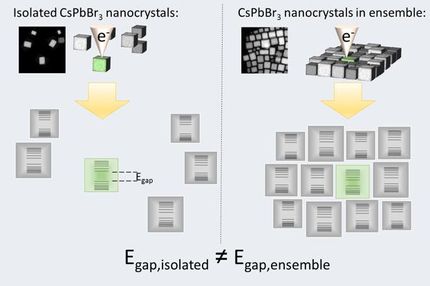
Atom swapping could lead to ultra-bright, flexible next generation LEDs
An international group of researchers has developed a new technique that could be used to make more efficient low-cost light-emitting materials which are flexible and can be printed using ink-jet techniques.

An international group of researchers has developed a new technique that could be used to make more efficient low-cost light-emitting materials which are flexible and can be printed using ink-jet techniques. The researchers, led by the University of Cambridge and the Technical University of Munich, found that by swapping one out of every one thousand atoms of one material for another, they were able to triple the luminescence of a new material class of light emitters known as halide perovskites.
Ella Maru Studio
The researchers, led by the University of Cambridge and the Technical University of Munich, found that by swapping one out of every one thousand atoms of one material for another, they were able to triple the luminescence of a new material class of light emitters known as halide perovskites.
This 'atom swapping', or doping, causes the charge carriers to get stuck in a specific part of the material's crystal structure, where they recombine and emit light. The results, reported in the Journal of the American Chemical Society, could be useful for low-cost printable and flexible LED lighting, displays for smartphones or cheap lasers.
Many everyday applications now use light-emitting devices (LEDs), such as domestic and commercial lighting, TV screens, smartphones and laptops. The main advantage of LEDs is they consume far less energy than older technologies.
Ultimately, also the entirety of our worldwide communication via the internet is driven by optical signals from very bright light sources that within optical fibres carry information at the speed of light across the globe.
The team studied a new class of semiconductors called halide perovskites in the form of nanocrystals which measure only about a ten-thousandth of the thickness of a human hair. These 'quantum dots' are highly luminescent materials: the first high-brilliance QLED TVs incorporating quantum dots recently came onto the market.
The Cambridge researchers, working with Daniel Congreve's group at Harvard, who are experts in the fabrication of quantum dots, have now greatly improved the light emission from these nanocrystals. They substituted one out of every one thousand atoms with another - swapping lead for manganese ions - and found the luminescence of the quantum dots tripled.
A detailed investigation using laser spectroscopy revealed the origin of this observation. "We found that the charges collect together in the regions of the crystals that we doped," said Sascha Feldmann from Cambridge's Cavendish Laboratory, the study's first author. "Once localised, those energetic charges can meet each other and recombine to emit light in a very efficient manner."
"We hope this fascinating discovery: that even smallest changes to the chemical composition can greatly enhance the material properties, will pave the way to cheap and ultrabright LED displays and lasers in the near future," said senior author Felix Deschler, who is jointly affiliated at the Cavendish and the Walter Schottky Institute at the Technical University of Munich.
In the future the researchers hope to identify even more efficient dopants which will help making these advanced light technologies accessible to every part of the world.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

From Paper to Digital - Management of Weighing Data -
Daunorubicin

Converting PFAS “forever chemicals” into valuable compounds - Scientists develop a new method to incorporate harmful perfluoroalkenes into N-heterocyclic carbene ligands
GV_Prasad
Methandrostenolone
Category:International_Technology_Roadmap_for_Semiconductors_lithography_nodes
Sucrose_gradient_centrifugation
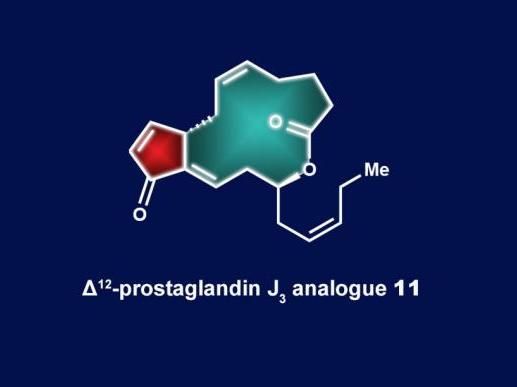
Rice University lab simplifies total synthesis of anti-cancer agent
Biodegradation
Johann_Böhm