CO2 Catalysis Made More Accessible
An economical alloy-based aerogel as electrocatalyst for carbon fixation
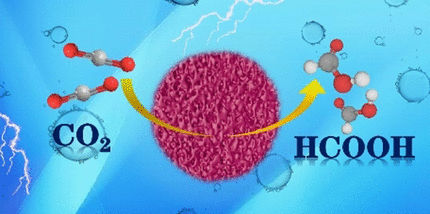
Many industrial processes emit carbon dioxide into the atmosphere. Unfortunately, however, current electrochemical separation methods are expensive and consume large amounts of power. They also require expensive and rare metals as catalysts. A study in the journal Angewandte Chemie describes a new aerogel electrocatalyst formed from an inexpensive metal alloy, which enables highly efficient electrochemical conversion of carbon dioxide. The main product is formic acid, which is a nontoxic basic chemical.
© Wiley-VCH
Capturing and chemically fixing carbon dioxide from industrial processes would be a huge step towards carbon neutrality. To prevent the notorious greenhouse gas from escaping into the air, it can be compressed and stored. Another option is electrochemical conversion to give other carbon compounds.
However, due to high power consumption and the cost of catalysts, electrochemical separation methods cannot be used on an industrial scale. This prompted Tianyi Ma of Swinburne University of Technology in Hawthorn, Australia, and colleagues to investigate replacement materials. The electrocatalysts currently used are made from precious metals such as platinum and rhenium. They catalyze electrochemical carbon fixation processes very efficiently, but they are also very expensive.
The authors discovered that the nonprecious metals tin and bismuth can form aerogels, which are incredibly light materials with particularly promising catalyst properties. Aerogels contain an ultraporous network that promotes electrolyte transport. They also offer up abundant sites where the electrochemical processes can take place.
To produce the aerogels, the team mixed a solution of bismuth and tin salts with a reducing agent and a stabilizer. Simply stirring this mixture led to a stable hydrogel of a bismuth–tin alloy after six hours at room temperature. A straightforward freeze-drying process produced the aerogel, formed of loosely interwoven and branched nanowires.
The authors found the bimetallic aerogel performed outstandingly well for carbon dioxide conversion. Compared to pure bismuth, pure tin, or the non-freeze-dried alloy, a significantly higher current density was observed. The conversion took place with an efficiency of 93%, which was at least as efficient, if not more so, than the standard materials currently used, indicating a low-waste process.
The process showed “excellent selectivity and stability for the production of formic acid under normal pressure at room temperature.” The only byproducts were carbon monoxide and hydrogen formed in miniscule amounts. The authors explain that this selectivity and stability was a result of the energy conditions at the surface of the alloy. Here, the carbon dioxide molecules accumulate in such a way that the carbon atom is free to bind hydrogen atoms from water molecules. This gives formic acid as the favored product.
This research hints at positive future prospects for other combinations of metals. It is likely that other nonprecious metals would convert to aerogels, forming inexpensive, nontoxic, and highly efficient catalysts for electrochemical carbon dioxide reduction.
Original publication
Original publication
Zexing Wu et al.; "Engineering Bismuth–Tin Interface in Bimetallic Aerogel with a 3D Porous Structure for Highly Selective Electrocatalytic CO2 Reduction to HCOOH"; Angewandte Chemie International Edition; 2021
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Segelerite
Clariant divests Detergents & Intermediates business
Quinuclidone
2024_aluminum
Organoborane

GE Osmonics SAS - Le Mee sur Seine, France
DSM invests in biomaterials company Oxford Performance Materials
VSEPR & Shapes of Molecules
Nanogel
Category:Niobium_compounds