Optically Active Defects Improve Carbon Nanotubes
Scientists achieve defect control with a new reaction pathway
The properties of carbon-based nanomaterials can be altered and engineered through the deliberate introduction of certain structural “imperfections” or defects. The challenge, however, is to control the number and type of these defects. In the case of carbon nanotubes – microscopically small tubular compounds that emit light in the near-infrared – chemists and materials scientists at Heidelberg University led by Prof. Dr Jana Zaumseil have now demonstrated a new reaction pathway to enable such defect control. It results in specific optically active defects – so-called sp3 defects – which are more luminescent and can emit single photons, that is, particles of light. The efficient emission of near-infrared light is important for applications in telecommunication and biological imaging.
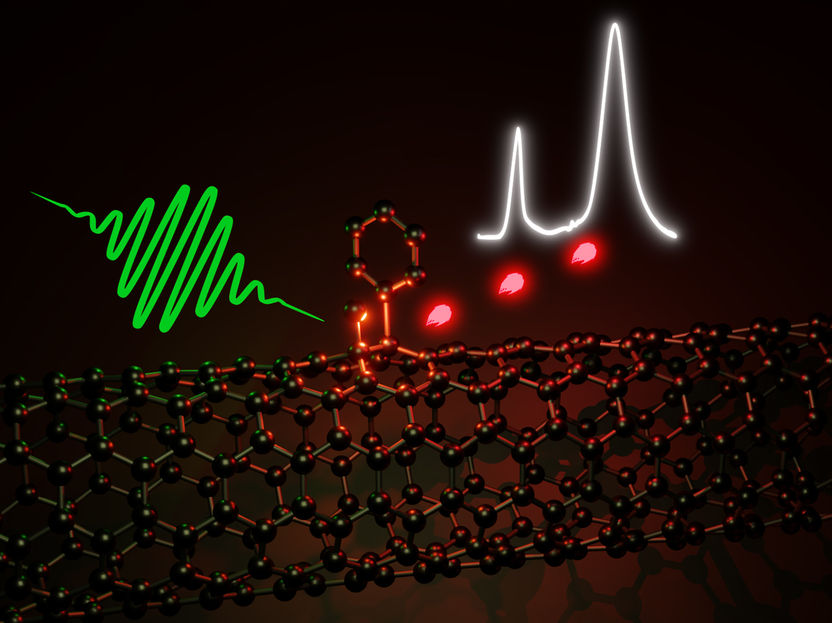
The optical properties of carbon nanotubes, which consist of a rolled-up hexagonal lattice of sp2 carbon atoms, can be improved through defects.
Simon Settele (Heidelberg)
Usually defects are considered something “bad” that negatively affects the properties of a material, making it less perfect. However, in certain nanomaterials such as carbon nanotubes these “imperfections” can result in something “good” and enable new functionalities. Here, the precise type of defects is crucial. Carbon nanotubes consist of rolled-up sheets of a hexagonal lattice of sp2 carbon atoms, as they also occur in benzene. These hollow tubes are about one nanometer in diameter and up to several micrometers long.
Through certain chemical reactions, a few sp2 carbon atoms of the lattice can be turned into sp3 carbon, which is also found in methane or diamond. This changes the local electronic structure of the carbon nanotube and results in an optically active defect. These sp3 defects emit light even further in the near-infrared and are overall more luminescent than nanotubes that have not been functionalised. Due to the geometry of carbon nanotubes, the precise position of the introduced sp3 carbon atoms determines the optical properties of the defects. “Unfortunately, so far there has been very little control over what defects are formed,” says Jana Zaumseil, who is a professor at the Institute for Physical Chemistry and a member of the Centre for Advanced Materials at Heidelberg University.
The Heidelberg scientist and her team recently demonstrated a new chemical reaction pathway that enables defect control and the selective creation of only one specific type of sp3 defect. These optically active defects are “better” than any of the previously introduced “imperfections”. Not only are they more luminescent, they also show single-photon emission at room temperature, Prof. Zaumseil explains. In this process, only one photon is emitted at a time, which is a prerequisite for quantum cryptography and highly secure telecommunication.
According to Simon Settele, a doctoral student in Prof. Zaumseilʼs research group and the first author on the paper reporting these results, this new functionalisation method – a nucleophilic addition – is very simple and does not require any special equipment. “We are only just starting to explore the potential applications. Many chemical and photophysical aspects are still unknown. However, the goal is to create even better defects.”
This research is part of the project “Trions and sp3-Defects in Single-walled Carbon Nanotubes for Optoelectronics” (TRIFECTs), led by Prof. Zaumseil and funded by an ERC Consolidator Grant of the European Research Council (ERC). Its goal is to understand and engineer the electronic and optical properties of defects in carbon nanotubes.
“The chemical differences between these defects are subtle and the desired binding configuration is usually only formed in a minority of nanotubes. Being able to produce large numbers of nanotubes with a specific defect and with controlled defect densities paves the way for optoelectronic devices as well as electrically pumped single-photon sources, which are needed for future applications in quantum cryptography,” Prof. Zaumseil says.
Original publication
Most read news
Original publication
S. Settele, F.J. Berger, S. Lindenthal, S. Zhao, A. Ali El Yumin, N.F. Zorn, A. Asyuda, M. Zharnikov, A. Högele & J. Zaumseil; "Synthetic control over the binding configuration of luminescent sp³-defects in single-walled carbon nanotubes"; Nature Communications; 9 April 2021.
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.


























































