The egg in the X-ray beam
Innovative time-resolved study reveals network formation and dynamics of proteins
Using DESY's X-ray light source PETRA III, a research team has analysed the structural changes in eggs during cooking. The study shows how the proteins in chicken egg whites unfold and crosslink during heating to form a solid structure. The innovative investigation method is interesting for the food industry as well as for the large field of protein analyses in research, as the cooperation of the groups of Frank Schreiber from the University of Tübingen and Christian Gutt from the University of Siegen together with researchers from DESY and from the European X-ray laser European XFEL has shown with two scientific publications in the journal "Physical Review Letters".
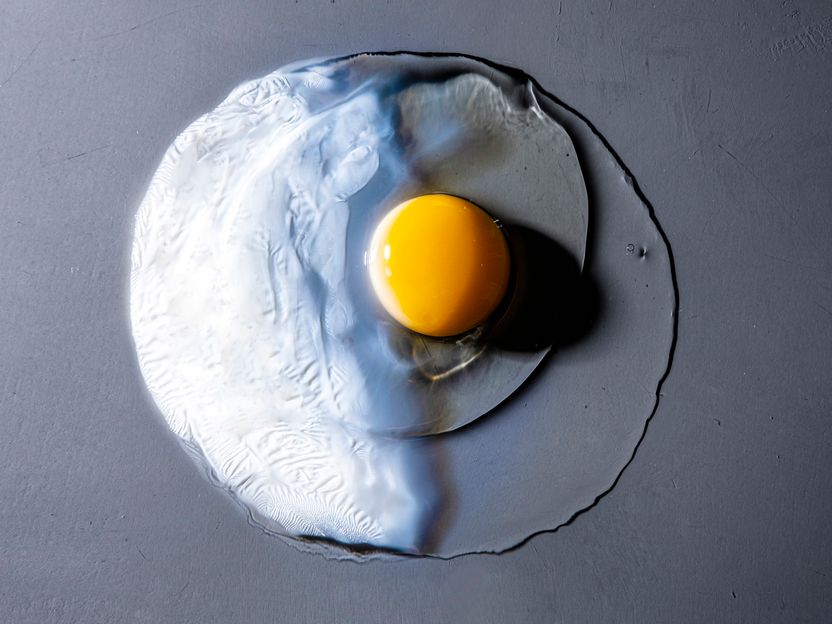
When heated, the proteins in the originally transparent chicken egg white form a tightly meshed, opaque network.
DESY
Eggs are among the most diverse ingredients for food. They can be gel, foam or comparatively solid and also serve as the basis for emulsions. At about 80 degrees Celsius, egg white ("albumen") becomes solid and also optically opaque. This is due to the fact that the proteins in the egg white form a network structure when heated. To study the exact molecular structure of egg whites, short-wavelength radiation such as X-rays, which penetrate the opaque protein and whose wavelength is no longer than the structures being studied, is required.
"To understand the structural change in detail, you need to study the phenomenon at the micrometer scale," explains the lead author of the first study, Alexander von Humboldt fellow Nafisa Begam from Schreiber's group. The researchers used X-ray photon correlation spectroscopy (XPCS) in a specific geometry so that the structure and dynamics of the proteins in the protein could be determined simultaneously.
For their experiments at the P10 measuring station at PETRA III, the scientists used a commercial chicken egg and filled the protein into a quartz tube with a diameter of 1.5 millimetres. "Inside, the egg white was heated in a controlled way while we analysed it with X-rays," reports co-author Fabian Westermeier from DESY. "The X-ray beam was thereby expanded to 0.1 by 0.1 millimeters, so that the radiation dose did not damage the protein structures."
The measurement shows the protein dynamics in the protein over about a quarter of an hour. According to the results, the protein network grew exponentially in the first nearly three minutes and reached a plateau after about five minutes, where almost no further protein linkages formed. The average mesh size of the protein network after this time was about 0.4 micrometers (thousandths of a millimeter).
In the second study, the team used the XPCS technique to investigate the self-assembly of protein solutions into protein-rich and protein-poor domains as an example of structure formation in cell biology. This allowed the temperature-dependent dynamics to be followed in a time-dependent manner. "At high protein concentration, mobility decreases, which slows down the evolution of phase separation. This is important for the particular dynamics of the system," reports lead author Anita Girelli from Schreiber's group.
The studies, funded by the German Federal Ministry of Education and Research (BMBF), not only reveal new details about the structural change in protein, but equally prove the investigation concept can be used for other samples, as the second study demonstrates. "The successful application of X-ray photon correlation spectroscopy opens up a new way to study the dynamics of biomolecules, which is essential to truly understand them," Schreiber emphasizes.
DESY is one of the world's leading particle accelerator centres, investigating the structure and function of matter - from the interplay of the smallest elementary particles, the behaviour of novel nanomaterials and vital biomolecules to the great mysteries of the universe. The particle accelerators and the detection instruments that DESY develops and builds at its sites in Hamburg and Zeuthen are unique tools for research: they generate the most powerful X-ray light in the world, bring particles to record energies and open new windows into the universe. DESY is a member of the Helmholtz Association, Germany's largest scientific organization, and is funded 90 percent by the Federal Ministry of Education and Research and 10 percent by the states of Hamburg and Brandenburg.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Steam_drum
Møller-Plesset_perturbation_theory
Twisting graphene into spirals - Researchers synthesize helical nanographene
The most exotic fluid has an unexpectedly low viscosity
Gramicidin

Sniffing out a better chemical sensor - Researchers at the NIST have created a new approach for “electronic noses”
Analytik Jena Delivers Instruments for Environmental Analysis to Poland
Corpuscularianism

Nanosphere at the quantum limit - Sensors could be immensely improved with nanospheres

Researchers discover carbon material with unique structure - "The material we synthesized is a hermaphrodite: for the first time, it forms a bridge between crystalline and amorphous, i.e. completely disordered, structures"