Gold-phosphorus nanosheets catalyzes nature gas to greener energy selectively
Water enables mild oxidation of methane to methanol on gold single-atom catalysts
Advertisement
Advances in hydraulic fracturing technology have enabled discovery of large reserves of natural gas which primarily contains methane, which is mainly burned directly and causing global warming potentially. Upgrading methane to greener energy such as Methanol through aerobic Oxidation is an ideal way to solve the problem and remain 100% atom economy.
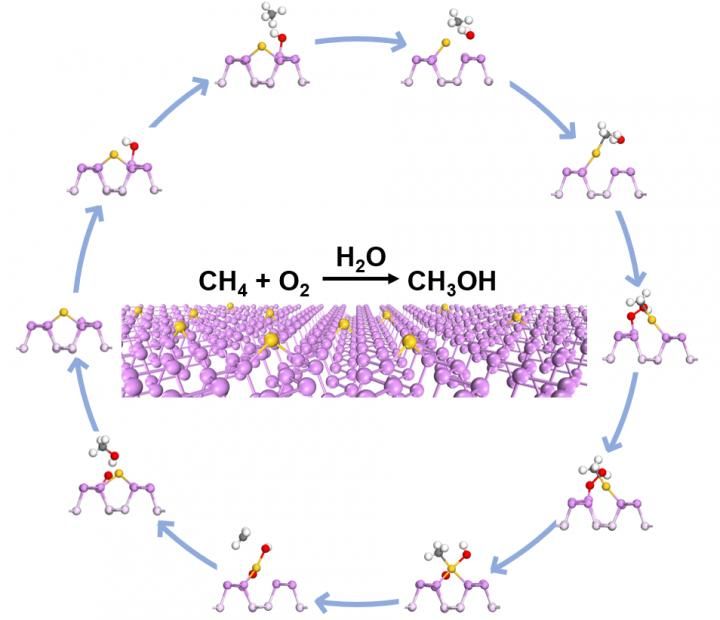
Schematic diagram of the reaction pathway for methane oxidation over Au1/BP nanosheets.
LUO Laihao
Yet the difficulties lie in activating methane and preventing methanol from over-oxidation. Methane takes a stable non-polar tetrahedral structure with high dissociation energy of C-H bond, which requires high energy to be activated. Meanwhile methanol can be easily over-oxidized to carbon dioxide during the process. The activation and directional transformation of methane is regarded as the "holy grail" of catalysis.
A recent work published on Nature Communications by research team led by Prof. ZENG Jie and LI Weixue from Hefei National Laboratory for Physical Sciences at the Microscale marks new progress. They designed and fabricated Au single atoms on black phosphorus (Au1/BP) nanosheets for methane selective oxidation into methanol under mild conditions with >99% selectivity.
Au1/BP nanosheets was able to catalyze methane oxidation reaction with oxygen as oxidant under irradiation conditions. Based on mechanistic studies, water and O2 were activated on Au1/BP nanosheets to form reactive hydroxyl groups and * OH radicals under light irradiation. The reactive hydroxyl groups enabled mild oxidation of methane into CH3* species, followed by oxidation of CH3* via * OH radicals into methanol.
Since water is consumed to form hydroxyl groups and produced via reaction of hydroxyl groups with methane, water is completely recycled and thus can also be regarded as a catalyst.
This study provides insight into the activation mechanism of oxygen and methane in methane selective oxidation, and offers a new understanding of the role of water in the reaction process.































































