Finding a better way to recover precious metals from electronic waste
"It's like being a metal whisperer. We make things go the way we want"
Inspired by nature's work to build spiky structures in caves, engineers at Iowa State University have developed technology capable of recovering pure and precious metals from the alloys in our old phones and other electrical waste.
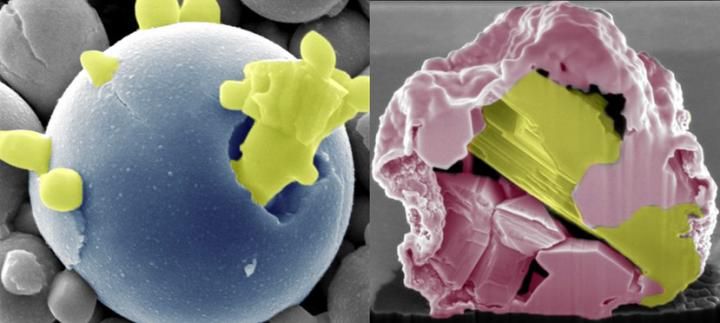
New technology developed by Iowa State engineers uses heat and oxidation to recover pure and precious metals from electronic waste. It works in two ways -- it can bring the most reactive components to the surface, forming stalagmite-like spikes (left); and it can leave the least reactive components in the core surrounded by metal-oxide spikes, creating a "ship-in-a-bottle" structure (right).
Photo courtesy of Martin Thuo/Iowa State University
Using controlled applications of oxygen and relatively low temperatures, the engineers say they can dealloy a metal by slowly moving the most reactive components to the surface where they form stalagmite-like spikes of metal oxides.
That leaves the least-reactive components in a purified, liquid core surrounded by brittle metal-oxide spikes "to create a so-called 'ship-in-a-bottle structure,'" said Martin Thuo, the leader of the research project and an associate professor of materials science and engineering at Iowa State University.
"The structure formed when the metal is molten is analogous to filled cave structures such as stalactites or stalagmites," Thuo said. "But instead of water, we are using oxidation to create these structures."
University startup funds and part of a U.S. Department of Energy Small Business Innovation Research grant supported development of the technology.
Thuo noted this project is the exact opposite of his research group's previous work to develop heat-free solder.
"With heat-free solder, we wanted to put things together," he said. "With this, we want to make things fall apart."
But not just fall apart any which way. Thuo and the engineers in his research group want to control exactly how and where alloy components fall apart, or dealloy.
"It's like being a metal whisperer," he said. "We make things go the way we want."
The engineers offered a more precise description in their paper: "This work demonstrates the controlled behavior of surface oxidation in metals and its potential in design of new particle structures or purification/dealloying. By tuning oxidation via temperature, oxidant partial pressure, time and composition, a balance between reactivity and thermal deformation enables unprecedented morphologies."
Those unprecedented forms and structures could be very useful.
"We need new methods to recover precious metals from e-waste or mixed metal materials," Thuo said. "What we demonstrate here is that the traditional electrochemical or high-temperature methods (above 1,832 degrees Fahrenheit) may not be necessary in metal purification as the metal's reactivity can be used to drive separation."
Thuo said the oxidation technology works well at temperatures of 500 to 700 degrees Fahrenheit. ("This is set in an oven and getting metals to separate," he said.)
Besides metal purification and recovery, this new idea could also be applied to metal speciation - the ability to dictate creation and distribution of certain metal components. One use could be production of complex catalysts to drive multi-stage reactions.
Let's say chemists need a tin oxide catalyst followed by a bismuth oxide catalyst. They'll start with an alloy with the bismuth oxide buried beneath the tin oxide. They'll run the reaction with the tin oxide catalyst. Then they'll raise the temperature to the point that the bismuth oxide comes to the surface as spikes. And then they'll run the reaction with the bismuth oxide catalyst.
Thuo credits development of the new technology to working with talented students and two collaborators.
"We built on this big idea very slowly," he said. "And working together, we were able to break into this knowledge gap."