Ultraheavy precision polymers
Controlled photoenzymatic RAFT polymerization of nonconjugated polymers
An environmentally friendly and sustainable synthesis of "heavyweight" polymers with very narrow molecular weight distributions is an important concept in modern polymer chemistry. Thanks to a new photoenzymatic process, Chinese researchers have been able to increase the range of possible monomers. As reported in the journal Angewandte Chemie, the researchers were able to obtain well-defined linear and star-shaped polymers with ultrahigh molecular weights.
© Wiley-VCH
Because many polymer properties depend heavily on molecular weight, it is desirable to have as narrow a molecular weight distribution as possible. Precision polymers with ultrahigh molecular weights (> 1 t/mol) would be interesting candidates for high-performance elastomers, low-concentration hydrogels, photonic materials, durable coatings, and flocking agents. However, such heavyweight polymers are not easy to produce with a uniform distribution of molecular weights. The radical polymerizations in widespread use are especially difficult to control in this respect. Modern methods, such as RAFT polymerization (RAFT: reversible addition-fragmentation chain transfer) offer a significantly higher degree of control by keeping the concentration of reactive radicals very small. A special agent reacts reversibly with the growing polymer chains to form a nonradical species. Whenever the intermediate dissociates, new active radicals are formed. This slows the reaction and results in longer, more uniform polymer chains.
Ultraheavy polymers with narrow weight distributions were previously only attainable from conjugated monomers, meaning compounds with at least two C=C double bonds separated by a single bond. It has never been possible to make such polymers from nonconjugated polymers whose vinyl group (-CH=CH(2)) is bound directly to a noncarbon atom.
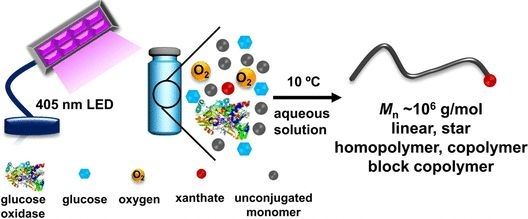
Zesheng An (Jilin University, Changchun) and Ruoyu Li (Shanghai University) have overcome this challenge with a simple, environmentally friendly, RAFT polymerization that is based on enzymatic photocatalysis. The enzyme glucose oxidase (GOx) oxidizes glucose with oxygen, reducing the flavin-containing cofactor FAD to FADH(?). The latter acts as a photocatalyst when irradiated with visible light, starting the radical chain reaction. GOx consumes the oxygen present in the solution--another advantage because oxygen disrupts conventional radical polymerizations and must be removed beforehand. The chain propagation agents they use are xanthates (sulfur-containing carboxylic acid derivatives).
The researchers attained well-defined linear and star-shaped polymers in nearly quantitative yield, as well as various copolymers with previously unattainable uniform ultrahigh molecular weights, starting from nonconjugated monomers. The reaction, which offers outstanding control over composition, molecular weight, and architecture, is simple to carry out and takes place under mild conditions (10 °C) in water.
Original publication
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.