Better catalysts for a sustainable bioeconomy
Get zeolite research out of the dead end
Researchers at the Paul Scherrer Institute PSI and from ETH Zurich want to make so-called zeolites more efficient. Today, these compounds are already indispensable additives in the chemical industry and have been used as catalysts in oil refineries since the 1960s. Now, in the journal Nature Materials, the researchers advocate paying more attention to the classic zeolites. These, they assert, would even have the potential to make a bioeconomy based on renewable resources possible.
Vitaly Sushkevich (left) and Manoj Ravi in the zeolite laboratory at PSI, holding a model of a standard zeolite.
Paul Scherrer Institut/Mahir Dzambegovic
To transform our fossil fuel-based economy into a sustainable bioeconomy, we must replace fossil resources with renewable raw materials. But petroleum, the starting material for numerous products of the chemical industry, cannot simply be exchanged for wood, maize, and straw, since plants consist of completely different kinds of molecules than "black gold". To power automobiles and enable the production of a broad range of plastics or drugs, renewable raw materials must first undergo a chemical conversion. Here help is provided by catalysts, that is, substances that drive chemical reactions or make them possible in the first place.
Extremely promising catalysts for this purpose are zeolites, scaffold-like compounds made of aluminium, oxygen, and silicon. Zeolites occur naturally – for example as minerals in rock formations – or are produced synthetically. They are among the most important catalysts in the chemical industry. Since the 1960s they have been used in oil refineries for cracking, the process of splitting long hydrocarbon chains into shorter ones. They are also used, for example, as ingredients in detergents, in water softening processes, and in heat reservoir systems.
Zeolites facilitate the transition to a bioeconomy by making it possible to convert biomass into molecules that industry desperately needs. However: "At this point, research on zeolites has reached a dead end," says Vitaly Sushkevich, a scientist in the Laboratory for Catalysis and Sustainable Chemistry at PSI. Together with colleagues at PSI and ETH Zurich, he wants to get zeolite research out of this dead end.
All aluminium is not the same
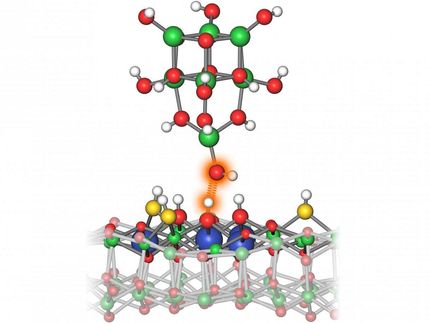
The problem: To develop catalysts for the bioeconomy, researchers worldwide are working on zeolites that also contain tin, titanium, or zirconium atoms. However, their performance cannot be increased any further. Therefore Sushkevich's team recommends turning back to the classic zeolites, which are only composed of silicon, aluminium, and oxygen. "They are very efficient catalysts," says Sushkevich. "The special thing is that they can be modified and adapted as required for specific purposes. You can even catalyse several chemical reactions one after the other." In this case, the desired product D is conveniently created from the starting material A through the intermediate steps B and C.
Aluminium atoms are an important element of these zeolites. Originally, these are firmly anchored in the zeolite scaffold. Through heating and other tricks, they can be released from this compound and thus put in a position to catalyse reactions that are important for the bioeconomy.
Doctoral candidate Manoj Ravi from ETH Zurich analysed the literature on this and found several inconsistencies. "The way the aluminium atoms catalyse reactions is evidently much more complicated than was previously thought," he says. For example, not all aluminium atoms are completely released from the scaffold compound. Instead, three different types of aluminium atoms coexist in such a zeolite: those that are still stuck in the scaffolding, those that are partially detached, and those that are completely detached. "It is important to distinguish these three types from each other and not to lump them together."
Understanding what's happening
PSI also synthesises zeolites itself and analyses their structures, for example with the help of the Swiss Light Source SLS. "Measurements at large research facilities and with other modern technologies help us better understand the structure of the important active centres," says Sushkevich. Active centres are the sites in a catalyst where the reaction takes place.
This approach could be helpful not only with the transition to a bioeconomy, but also in processing classic fossil resources, adds the chemist.