From greenhouse gas to valuable basic chemicals
Ionic liquids help to understand the electrocatalytic conversion of CO₂
The conversion of carbon dioxide into hydrocarbons and other basic chemicals is important if we are to have a sustainable economy in the future. Researchers at the TU Darmstadt and the Helmholtz Institute Erlangen-Nürnberg for Renewable Energy have now decoded major steps in electrochemical carbon dioxide conversion.
Etzold Lab
The conversion of carbon dioxide into hydrocarbons and other basic chemicals plays an important role on the road to a sustainable economy. One promising process is the electrochemical conversion of gas isolated from the air or from industrial waste and secondary streams to copper catalysts. Solar or wind power can be used as the energy source. This also offers the possibility of storing excess renewable energy in the form of chemical energy. However, the electrocatalytic conversion of carbon dioxide is a complex process, and the individual steps have not yet been clarified. "A deeper insight into the reaction mechanisms is essential in order to steer the implementation of carbon dioxide towards the desired target products," emphasises Professor Bastian J. M. Etzold of the Department of Chemistry at TU Darmstadt.
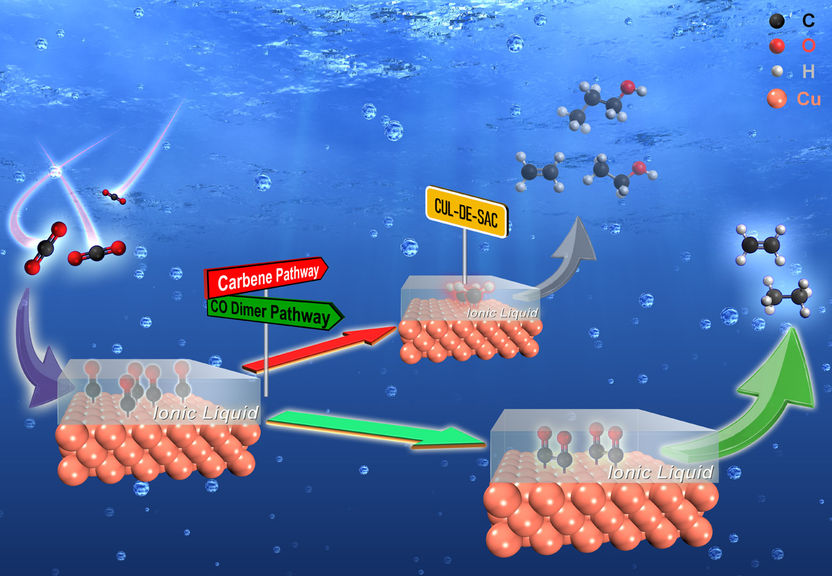
Together with the group of Professor Jan P. Hoffmann (Department of Materials and Earth Sciences at TU Darmstadt) and researchers at the Helmholtz Institute Erlangen-Nürnberg for Renewable Energy, Etzold and his colleagues have now decoded essential steps in electrochemical carbon dioxide conversion. And they did so with a trick, as they now report in the journal Angewandte Chemie International Edition: the scientists applied an ionic liquid to the copper catalyst that acted as a chemical trap. This allows intermediates of the electrochemical conversion to be intercepted and certain reaction steps to be prevented or slowed down. "We were able to use the resulting change in the product spectrum to simplify the complex response network and identify key steps," explained Professor Etzold. Among other things, the scientists were able to derive new findings on the conversion of carbon dioxide to the alcohols ethanol and propanol as well as to the hydrocarbons ethane and ethene.
The strategy is based on a concept called SCILL (solid catalyst with ionic liquid layer) that Etzold first published 13 years ago. To date, SCILL has been used e.g. to modify platinum catalysts for fuel cells. Applying the ionic liquid to the catalyst is easy, emphasises Etzold. "The method can be used in numerous laboratories and specialised test rigs, even under technically relevant conditions." Thanks to the variety of ionic liquids, the approach is also suitable for the study of other electrochemical reactions as well as for controlling the product range in electrocatalysis in general.
Original publication
Original publication
G.-R. Zhang, S.-D. Straub, L.-L. Shen, Y. Hermans, P. Schmatz, A.M. Reichert, J.P. Hofmann, I. Katsounaros and B.J.M. Etzold; "Probing CO2 Reduction Pathways in Copper Catalysts using Ionic Liquid as a Chemical Trapping Agent"; Angew. Chem. Int. Ed.; 2020
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.



























































