Template-Free Synthesis of Mesoporous β-MnO2 Nanoparticles
Way, shape and form: Synthesis conditions define the nanostructure of manganese dioxide
Scientists at Tokyo Institute of Technology explore a novel and simplistic method to synthesize manganese dioxide with a specific crystalline structure called β-MnO2. Their study sheds light on how different synthesis conditions can produce manganese dioxide with distinct porous structures, hinting at a strategy for the development of highly tuned MnO2 nanomaterials that could serve as catalysts in the fabrication of bioplastics.
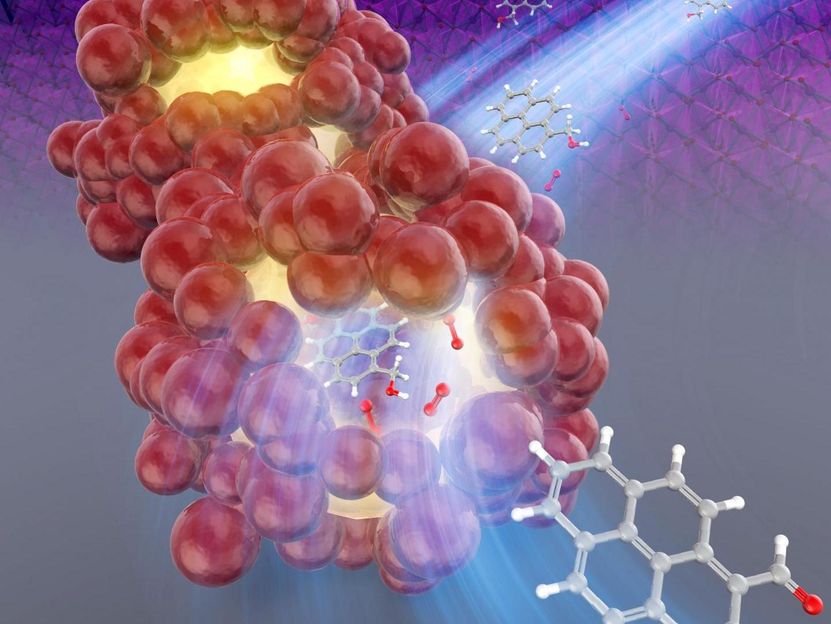
Acceleration of the chemical reaction by β-MnO2 catalyst in the nanospace of the particles.
Keiko Kamata, Tokyo Institute of Technology
Materials engineering has advanced to a point at which not only are we concerned about the chemical composition of a material, but also about its structure at a nanometric level. Nanostructured materials have recently drawn the attention of researchers from a variety of fields and for good reason; their physical, optical, and electrical characteristics can be tuned and pushed to the limit once methods to tailor their nanostructure are available.
Manganese dioxide (chemical formula MnO2) nanostructured metal oxide that can form many different crystalline structures, with applications across various engineering fields. One important use of MnO2 is as a catalyst for chemical reactions, and a particular crystalline structure of MnO2, called β-MnO2, is exceptional for the oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid (FDCA). Because FDCA can be used to produce environment-friendly bioplastics, finding ways to tune the nanostructure of β-MnO2 to maximize its catalytic performance is crucial.
However, producing β-MnO2 is difficult compared with other MnO2 crystalline structures. Existing methods are complicated and involve the use of template materials onto which β-MnO2 "grows" and ends up with the desired structure after several steps. Now, researchers from Tokyo Institute of Technology led by Prof. Keigo Kamata explore a template-free approach for the synthesis of different types of porous β-MnO2 nanoparticles.
Their method, described in their study published in ACS Applied Materials & Interfaces, is outstandingly simple and convenient. First, Mn precursors are obtained by mixing aqueous solutions and letting the solids precipitate. After filtration and drying, the collected solids are subjected to a temperature of 400°C in a normal air atmosphere, a process known as calcination. During this step, the material crystallizes and the black powder obtained afterwards is more than 97% porous β-MnO2.
Most notably, the researchers found this porous β-MnO2 to be much more efficient as a catalyst for synthesizing FDCA than the β-MnO2 produced using a more widespread approach called the "hydrothermal method." To understand why, they analyzed the chemical, microscopic, and spectral characteristics of β-MnO2 nanoparticles produced under different synthesis conditions.
They found that β-MnO2 can take on markedly different morphologies according to certain parameters. In particular, by adjusting the acidity (pH) of the solution in which the precursors are mixed, β-MnO2 nanoparticles with large spherical pores can be obtained. This porous structure has a higher surface area, thus providing better catalytic performance. Excited about the results, Kamata remarks: "Our porous β-MnO2 nanoparticles could efficiently catalyze the oxidation of HMF into FDCA in sharp contrast with β-MnO2 nanoparticles obtained via the hydrothermal method. Further fine control of the crystallinity and/or porous structure of β-MnO2 could lead to the development of even more efficient oxidative reactions."
What's more, this study provided much insight into how porous and tunnel structures are formed in MnO2, which could be key to extending its applications, as Kamata states: "Our approach, which involves the transformation of Mn precursors into MnO2 not in the liquid-phase (hydrothermal method) but under an air atmosphere, is a promising strategy for the synthesis of various MnO2 nanoparticles with tunnel structures. These could be applicable as versatile functional materials for catalysts, chemical sensors, lithium-ion batteries, and supercapacitors." Further studies like this one will hopefully allow us to one day harness the full potential that nanostructured materials have to offer.
Original publication
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.