Sweet or Sour Natural Gas
Polyimide membranes for the purification of natural gas
Advertisement
natural gas that contains larger amounts of hydrogen sulfide (H2S) and carbon dioxide (CO2) is termed sour gas. Before it can enter a pipeline, it must be “sweetened” by removal of its acidic impurities. Through fine tuning of the ratios of two molecular components, it is possible to produce tailored polyimide membranes that can purify sour gas with a wide range of compositions, as reported by researchers in the journal Angewandte Chemie.

Natural gas that contains larger amounts of hydrogen sulfide (H2S) and carbon dioxide (CO2) is termed sour gas. Before it can enter a pipeline, it must be “sweetened” by removal of its acidic impurities (symbolic image).
Photo by Mike Benna on Unsplash
© Wiley-VCH

The main component of natural gas is methane (CH4). The H2S and CO2 in sour gas react acidically with moisture, making them highly corrosive. In addition, H2S is highly toxic and poses a safety risk. Today, sweetening is usually achieved through very energy-intensive chemical scrubbing, which is not economically viable for gas with high concentrations of H2S and CO2. In addition, this process requires a large, complex apparatus that is impossible to use in remote or offshore facilities. Scalable, economical membrane separations represent an excellent alternative.
Membranes based on glassy polyimide polymers made of a special nitrogen- and oxygen-containing group demonstrate good separation efficiency. However, a fundamental understanding of the relationships between the structures of polyimides and their gas-transport properties in the presence of H2S has been lacking, impeding the design of advanced membranes. A team led by William J. Koros at the Georgia Institute of Technology (Atlanta, USA) has now taken on this subject.
Membrane separations are based on the fact that gases with higher solubility pass more easily through membrane materials; however, smaller gas molecules can also diffuse through membranes more easily. The challenge for sweetening lies in the fact that the separation of CO2 relies primarily on a size difference (CO2 is smaller than CH4), while the separation of the similarly sized H2S and CH4 depends on differences in solubility. In addition, glassy polyimide membranes begin to soften as they absorb more dissolved gas. This is favorable for the separation of H2S but unfavorable for the separation of CO2.
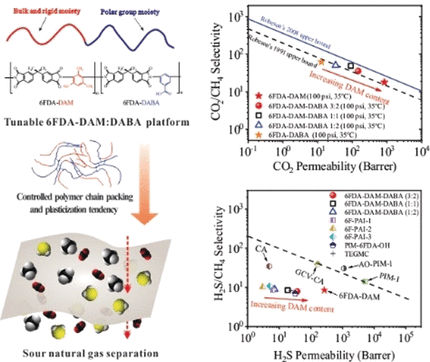
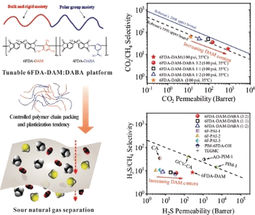
For their experiments, the researchers produced polyimides based on 6FDA (4,4'-(hexafluoroisopropylidene) diphthalic anhydride. They used two different 6FDA building blocks, which they polymerized in a variety of ratios. One building block (DAM) introduces a bulky trimethyl benzene group, which prevents the polymer chains from being densely packed. This increases both the gas permeability and the tendency to soften. The other building block (DABA) contains a polar benzoic acid group. This tightens the packing of the chains, decreasing permeability, but increases H2S solubility.
Higher proportions of DAM increase the permeability toward CO2, but also CH4, which decreases selectivity. In contrast, the selectivity with regard to H2S is barely affected. The more DAM included, the more the polymer softens, which is unfavorable for CO2 but favorable for H2S. By carefully adjusting the relative amounts of the building blocks, the packing of the polymer chains and the tendency to plasticize can be balanced to produce membranes that simultaneously and efficiently separate out both H2S and CO2. This makes it possible to tailor membranes for different natural gas compositions.