Molecular twist makes one catalyst useful for three hydrogen applications
"In a way, we've created a molecule with a steering wheel on it"
Scientists from Kyushu University and Kumamoto University in Japan have developed a new catalyst capable of assisting three key reactions for using hydrogen in energy and industry. Inspired by three types of enzymes in nature, this research can help elucidate unknown relationships among catalysts, paving the way for efficient use of hydrogen gas as a next-generation energy source in the future.
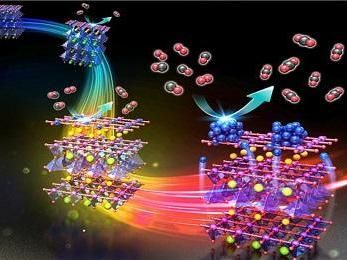
By turning the red, blue, and green positions of one molecule like turning the steering wheel of a car, a single molecule developed by researchers in Japan can be turned into three types of catalysts for fuel cells, hydrogen production, or hydrogenation. The new insights from this work help unravel relationships between three catalysts that were previously intertwined.
Kyushu University
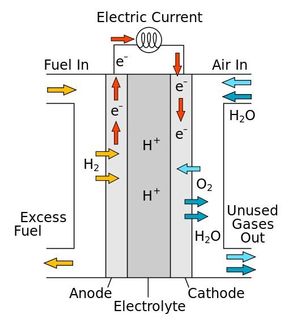
Producing only water when used in a fuel cell to generate electricity, hydrogen holds great promise as a clean energy source to meet environmental challenges faced around the globe. One key for establishing hydrogen as a next-generation energy source is the development of catalysts--chemicals that assist and accelerate reactions without being consumed in the process--that help use it efficiently.
Catalysts play a role not only in splitting hydrogen molecules to generate electricity in fuel cells but also in putting hydrogen atoms together to form the fuel. Hydrogen also has many applications in the chemical industry, often being attached to molecules through the process of hydrogenation to modify their properties.
Nature has already developed its own set of biological catalysts, known as enzymes, capable of these same fundamental reactions. However, each of these three reactions requires a different type of enzyme, and these hydrogenase enzymes can be grouped by the metals they contain: an atom each of nickel and iron, two atoms of iron, or a single atom of iron.
Taking inspiration from nature, research teams led by Seiji Ogo from Kyushu University and Shinya Hayami from Kumamoto University now report in the journal Science Advances that a single catalyst can perform all three roles.
"Looking closely at key structures of the three types of hydrogenase enzymes in nature, we were able to design a molecule that could mimic all of these structures depending on where hydrogen attaches to it," said Ogo, professor of Kyushu University's Department of Chemistry and Biochemistry.
The catalyst the scientists developed contains nickel and iron as the key metals. Depending on reaction conditions, hydrogen atoms will connect to the molecule in a slightly different way, leading to a twisting of the molecule that puts it in a configuration best suited for one of the three types of reactions.
While the enzymes in nature rely on different sets of metals to accomplish these reactions, the newly developed catalyst takes advantage of the molecular twist being enough to switch between structures similar to those of the three types of enzymes, thereby obtaining similar functions without changing the metals.
"In a way, we've created a molecule with a steering wheel on it," explains Ogo. "By turning the steering wheel and twisting parts of the molecule, we can turn it into three different types of catalysts--one for fuel cells, one for hydrogen production, and another for hydrogenation."
"This has allowed us to unravel three functions that were previously intertwined."
While the molecule may not be suitable for practical applications at present, it points toward the possibility of developing a single catalyst with multiple uses. More importantly, the better understanding of the catalytic processes afforded by this molecule can give crucial insight into natural enzymes and the development of future catalysts for realizing a hydrogen-powered society.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
BASF and GS Engineering and Construction intend to jointly develop modular solutions for Carbon Capture - One-stop solution from specialized engineering services, carbon capture, project financing to storage and utilization
Thermometer

New approach improves ability to predict metals' reactions with water
Humanized_antibody
Neptunism
STRIDE_(protein)