How to Gently Caress Atoms: A single oxygen atom is used as a highly sensitive sensor
How can surfaces be studied as gently as possible on an atomic scale?
oxygen is highly reactive. It accumulates on many surfaces and determines their chemical behavior. At the Vienna University of Technology, scientists study the interaction between oxygen and metal oxide surfaces, which play an important role in many technical applications - from chemical sensors and catalysts to electronics.
Martin Setvin (l) and Igor Sokolovic in the lab
Copyright: TU Wien
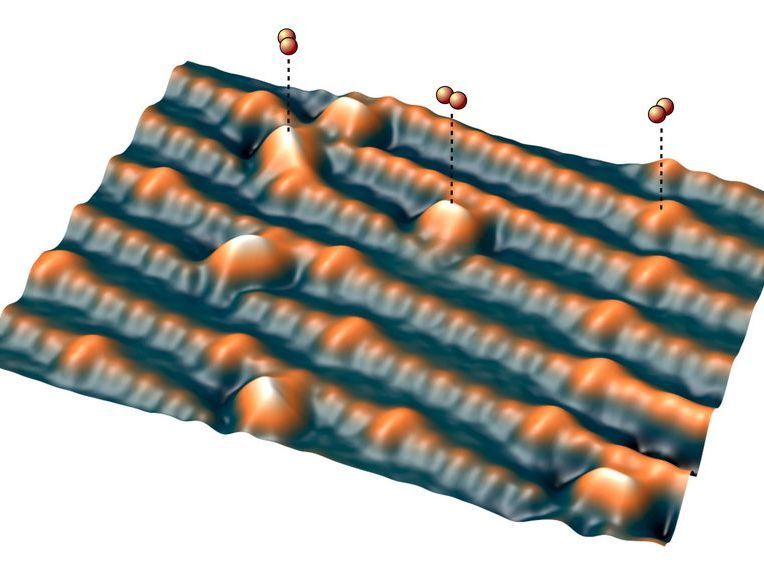
Oxygen molecules are adsorbed on the surface (orange)
Copyright: TU Wien

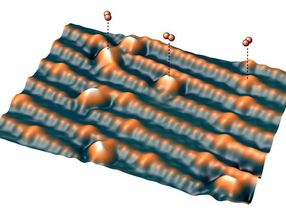
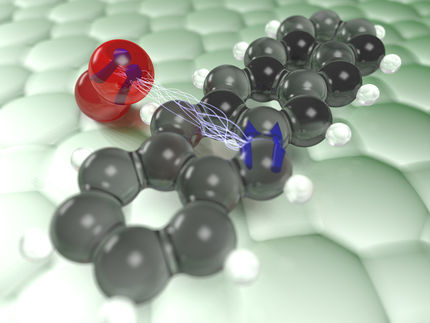
However, it is extremely difficult to study oxygen molecules on the metal oxide surface without altering them. At TU Wien, this has now been achieved with a special trick: a single oxygen atom is attached to the tip of an atomic force microscope and then it is gently guided across the surface. The force between the surface and the oxygen atom is measured, and an image is taken with extremely high resolution.
Different Kinds of Oxygen
"In recent years, quite a bit of research has been done on how oxygen attaches to metal oxide surfaces," says Prof. Martin Setvin from the Institute of Applied Physics at TU Wien. "Do O2 molecules remain intact, or are they broken down into single atoms? Or could it be possible that so-called tetraoxygen forms, a complex of four atoms? Such questions are important to understand chemical reactions on the metal oxide surface.”
Unfortunately, it is not easy to take a picture of these atoms. Scanning tunneling microscopes are often used to image surfaces atom by atom. A fine tip is passed over the sample at an extremely short distance, so that individual electrons can pass between the sample and tip. The tiny electrical current that results is measured. However, this method cannot be used for oxygen molecules - they would become electrically charged and completely change their behavior.
The Vienna scientists used an atomic force microscope instead. Here too, a thin tip is moved across the surface. In this case, no current flows, but the force that acts between the tip and the surface is measured. A special trick was decisive - the functionalization of the tip: "A single oxygen atom is first captured by the tip of the atomic force microscope and then moved across the surface," explains Igor Sokolovic. The oxygen atom thus serves as a highly sensitive probe to examine the surface point by point.
Since no current flows and the oxygen atom never comes into full contact with the surface, this method is extremely gentle and does not change the atoms on the metal oxide surface. In this way, the geometry of the oxygen deposits on the metal oxide can be examined in detail.
A Versatile Method
"This functionalization of the tip by placing a very specific atom on it has been developed in recent years, and we are now showing for the first time it can be applied to metal oxide surfaces," says Setvin.
It turns out that the oxygen molecules can be attached to the metal oxide in different ways - either on the titanium atoms at the surface or at certain positions, where an oxygen atom is missing. Depending on the temperature, the oxygen molecules can then split into two individual oxygen atoms. However, no tetraoxygen -- a hypothetical complex of four oxygen atoms -- was found.
"The titanium oxide surfaces that we examine in this way are a prototype case to put this method to test," explains Martin Setvin. "But the insights that we gain from our experiments also apply to many other materials." Microscopy with a functionalized tip in an atomic force microscope is a versatile method for imaging a surface structure with atomic resolution without destruction and without electronic change.
Original publication
M. Setvin et al., "Resolving the adsorption of molecular O2 on the rutile TiO2(110) surface by non-contact atomic force microscopy," PNAS (2020)
Original publication
M. Setvin et al., "Resolving the adsorption of molecular O2 on the rutile TiO2(110) surface by non-contact atomic force microscopy," PNAS (2020)
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
High-resolution atomic imaging of specimens in liquid by TEM using graphene liquid cell - Looking into specimens on an atomic level in liquids, and understanding atomic processes so far regarded impossible

On-site detection of hazardous substances
Software for the discovery of new crystal structures
Covestro generates strong volume growth in a continuing challenging environment - New circular economy program launched
Arkema Upgrades its Price Increases for High Performance Polyamides
20-hydroxyecdysone
A new way to make sheets of graphene
Pharmacy

Green hydrogen: A cage structured material transforms into a performant catalyst - Very interesting class of materials for electrocatalysts discovered?
Watching lithium in real time could improve performance of EV battery materials - Researchers tracked the movement of lithium ions inside a promising new battery material