Is the simplest chemical reaction really that simple?
Uncovered mechanism leads to clear quantum interference and verifies that Nature does "play dice"
Most people think that quantum theory, which describes the motion of molecules and atomic and subatomic particles, is counterintuitive, since quantum mechanics describes behavior at odds with classical mechanics. Even Albert Einstein, who never accepted quantum mechanics, famously said that "He (God or Nature) does not play dice" - meaning that the laws of physics do not surrender to uncertainty or chance as implied by quantum theory.
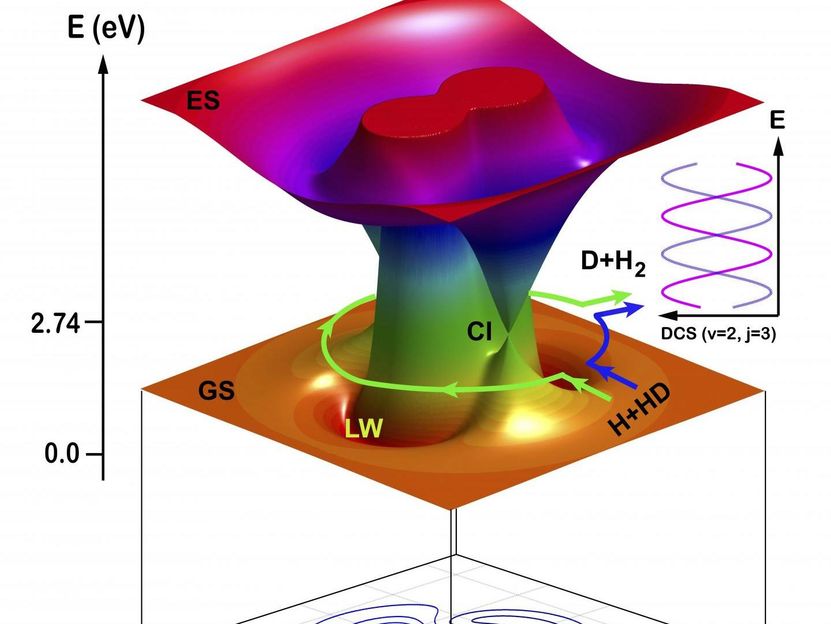
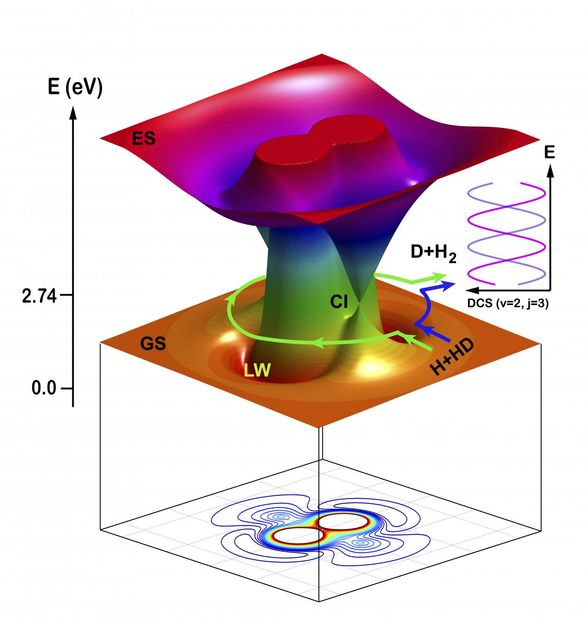
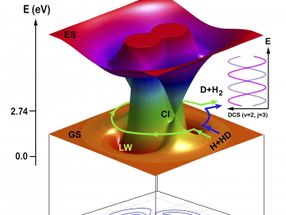
Schematic illustration of the two topological pathways in the H + HD to H2 + D reaction: direct abstraction reaction (counter-clockwise) path and the roaming insertion reaction (clockwise) path.
DICP
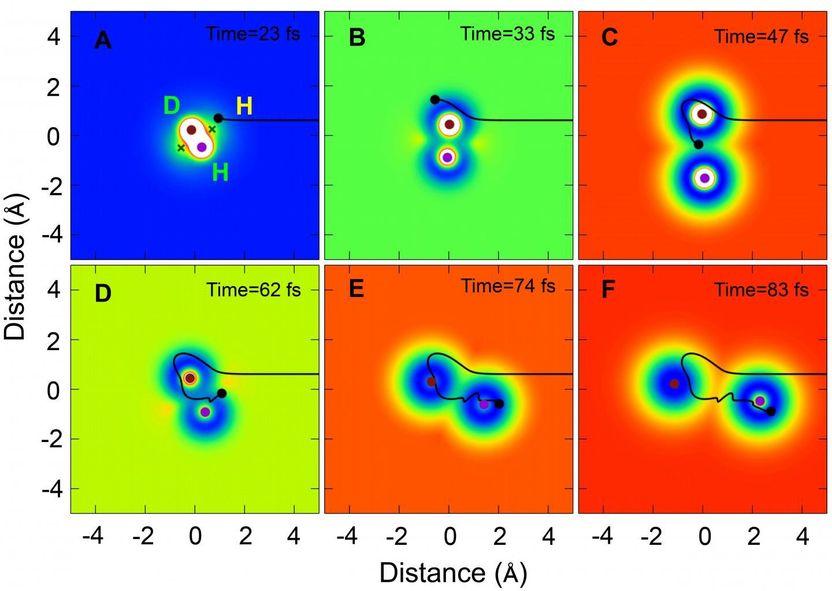
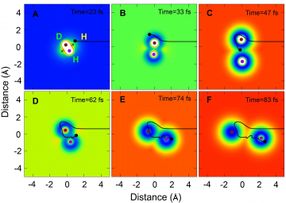
A representative trajectory of the H + HD to H2 + D reaction in certain scattering angle (backward scattering direction) via the roaming mechanism moving with time in Cartesian coordinate.
DICP
A chemical reaction sometimes occurs in an odd way, since in microscopic view the progress of a reaction is governed by the quantum theory.
New research by scientists at the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has shown, surprisingly, in the simplest, well-studied reaction, there is still uncovered mechanism. It leads to clear quantum interference and verifies again that Nature does "play dice".
The reaction in question is H + HD → H2 + D. In the study, published in Science on May 15, groups led by Profs. YANG Xueming, ZHANG Donghui, SUN Zhigang and XIAO Chunlei of DICP discovered a new kind of quantum interference in this simple reaction.
In physics, interference is the combination of two or more waveforms to form a resultant wave, in which the displacement is either reinforced or canceled. Quantum interference can happen between particles that arrive at the same position or quantum state but by different paths.
Since a chemical reaction is essentially a collision and scattering process involving atoms and/or molecules, we can expect interference phenomena to occur in a chemical reaction.
Among all chemical reactions, the H + H2 reaction and its isotopologues are the simplest ones. This reaction only involves three electrons; thus it is convenient to deal with accurate quantum chemistry to calculate the interaction energy involving the three atoms.
Last year, DICP researchers found strong and regular oscillations as a function of energy at certain scattering angle of the product H2 during the H + HD reaction in particular rovibrational states.
Actually, similar oscillations have been observed in other reactions, but they are not as regular as those in the H + HD reaction. The physical origin of such oscillations remains unclear.
To understand this interesting phenomenon, the researchers conducted a combined theoretical and experimental study of the H + HD reaction.
Experimentally, by improving the crossed molecular beam apparatus, they recorded reactive scattering signals at certain scattering angle as a function of relative high energy.
They further developed quantum dynamics methods by applying topological theory to analyze the paths through which the reaction proceeded. Topological theory revealed that the observed regular oscillations resulted from interference between products generated via two different paths.
The researchers analyzed the reaction dynamics mechanisms using quasi-classical trajectory (QCT) theory. The results showed that the reaction proceeded via one path using the traditional direct extraction mechanism, i.e., the incoming H atom collided with the H atom in the diatomic reactant HD molecule and extracted it to form a new chemical bond of H2.
The reaction also proceeded via another path using a new roaming mechanism. The snapshots from the QCT theory for the roaming mechanism show that the incoming H atom initially approached the HD molecule via the conical intersection (Cl) region in the direction of the D atom end, and then roamed around the D atom in HD. When the incoming H atom approached the CI region, the HD bond started to stretch, making it possible for the roaming H atom to insert itself into the stretched HD molecule. The incoming H atom then formed a new chemical bond with the H atom in HD.
The products (H2) from these two paths were scattered into the same scattering angle, where quantum interference occurred.
Moreover, the probability for such an unusual roaming mechanism to occur is quite low - only about 0.3% of all reactions.
This work once again demonstrates the quantum nature of a chemical reaction at the microscopic level. It also reveals that chemical reactions are complicated.
Even the simple reaction H + HD → H2 + D, which has been studied for decades, has a small probability of employing unexpected mechanisms.
In life, many big events are triggered by small-probability events. Who can guarantee that a reaction mechanism of such small probability will not lead to surprising results?