Nitrogen compounds with surprising structures discovered
Valuable starting points for the design and synthesis of new high-tech materials
Advertisement
nitrides are nitrogen compounds with technologically highly attractive properties. They therefore have the potential for widespread application in microelectronics, optoelectronics, and as ceramic materials. Researchers at the University of Bayreuth have now discovered unusual nitrides in high-pressure experiments. Nitrogen and metal atoms combine under very high pressures to form porous crystal structures with channels in which nitrogen molecules become embedded. The findings published in the journal „Angewandte Chemie International Edition” offer valuable starting points for the design and synthesis of new high-tech materials.
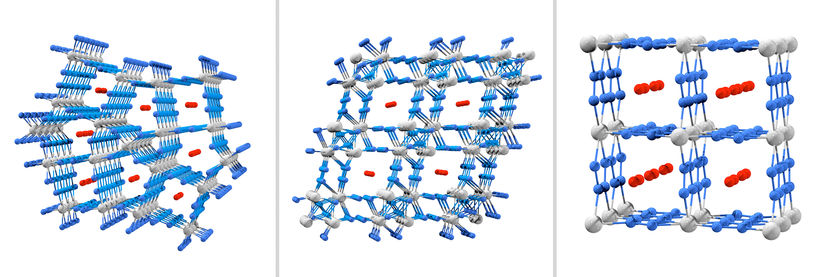
Metallic inorganic framework structures: Os₅N₂₈, Hf₄N₂₀ and WN₈ with the transition metals osmium, hafnium and tungsten (from left to right). The nitrogen atoms are blue, metal atoms yellow, and the nitrogen molecules in the interstices are red.
Maxim Bykov
Counter-intuitive: high pressure creates cavities
It's an everyday experience: the stronger the pressure you put on an object from all sides, the more it is compressed. The volume decreases, hollow spaces inside disappear. Yet it is precisely this experience that the new high-pressure experiments at the University of Bayreuth contradict. At a compression pressure of around one million atmospheres, such as exists around 2,500 kilometres below the Earth’s surface, porous framework structures are formed from nitrogen atoms and the atoms of a metal. Here, nitrogen atoms build up zigzag chains, for example. Nitrogen molecules (N₂) penetrate into the cavities of the new crystals. The metals used in the experiments were hafnium (Hf), tungsten (W), and osmium (Os). These belong to the group of transition metals due to their positions in the periodic table of elements.
High pressure prompts nitrogen to bond
Under the normal pressures and temperatures we are familiar with on Earth, nitrogen molecules are very unwilling to react with elements and compounds. “It is therefore fascinating to observe how radically the binding behaviour of nitrogen changes under high pressure. Complex framework structures are formed which contain different types of chemical bonds. In any case, these structures are porous - which is very unusual when you consider, for example, how layered graphite transforms into compact and very hard diamond under high pressure", explains Prof. Dr. Natalia Dubrovinskaia from the Laboratory for Crystallography at the University of Bayreuth, who was significantly involved in the new study.
The appearance of the complex framework structure which is created in each individual case depends crucially on the choice of transition metal. In principle, this means that the synthesis of the nitrides can be controlled in a targeted manner - at least under the high pressures that can be produced in the laboratory.
"In view of the growing technological importance of nitrides, for example for electronics and energy storage, our new study offers numerous prospects for the development of new high-tech materials," says Dr. Maxim Bykov, first author of the study, who completed his doctorate at the Laboratory for Crystallography at the University of Bayreuth, and worked until 2019 as a postdoc at the Bavarian Research Institute of Experimental Geochemistry & Geophysics (BGI) of the University of Bayreuth.