Unique structural fluctuations at ice surface promote autoionization of water molecules
Direct experimental evidence for markedly enhanced surface proton activity inherent to water ice
water ice is one of the most abundant solid substances in nature and hydrated protons on the ice surfaces critically influence physical and chemical properties of ices. Hydrated protons are easily doped into the hydrogen-bond (HB) networks when acidic impurities are present. In contrast, in pure water molecular systems, they are generated solely by the thermal ionization of water molecules (H2O⇆H+hyd + OH-hyd). Therefore, the proton activity inherent to water ice is determined by the amount and mobility of hydrated protons derived from the autoionization.
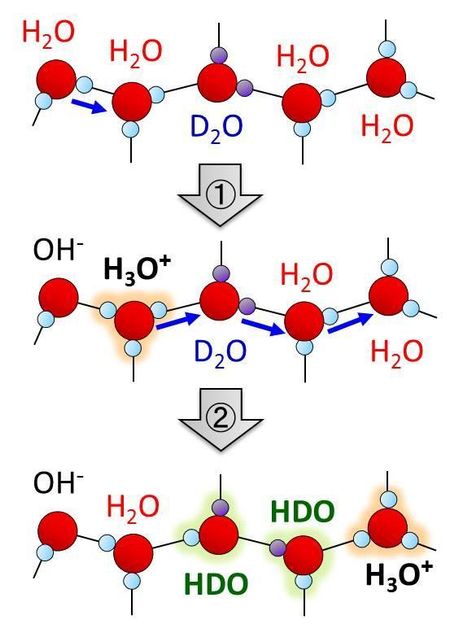
Schematic illustration of H/D isotope exchange process of water molecules induced by autoionization and subsequent proton transfer.
NINS/IMS
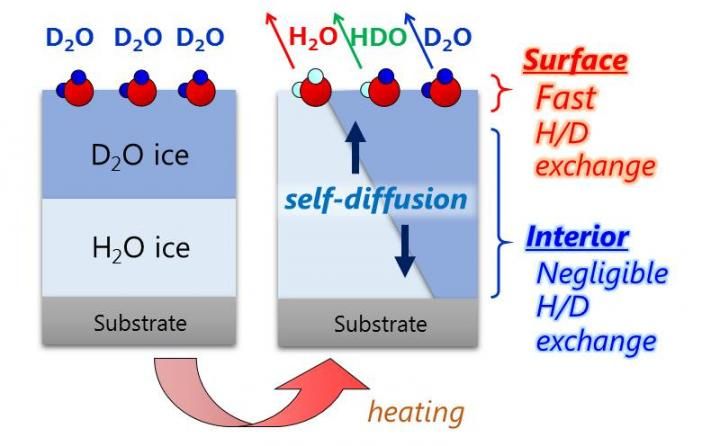
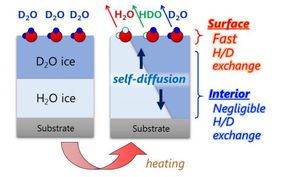
Simultaneous observation of the H/D isotopic exchange of water molecules at the surface and in the interior of well-defined double-layer ice films composed of H2O and D2O.
NINS/IMS
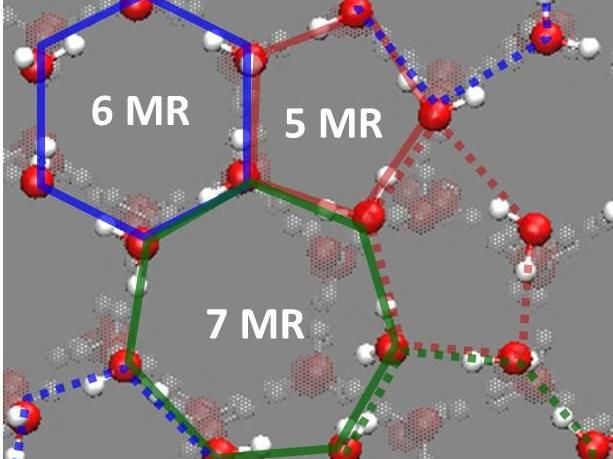
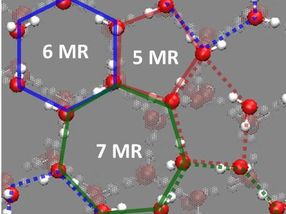
Snapshots of unique hydrogen-bond (HB) structure derived from cooperative surface relaxation and fluctuation at the topmost surface layer of crystalline ice at
NINS/IMS
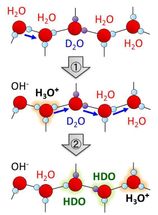
Considerable discussions have been made, yet not been settled, on whether the activity of hydrated protons is substantially enhanced at the surface of water ice. This is crucially important problem for understanding the impact of ice surface ubiquitous in nature on a wide variety of heterogeneous phenomena, such as charge generation, separation and trapping in a thunder storm, photochemical destruction of the earth's ozone layer, and even the molecular evolution in space, etc.
Very recently, researchers led by Toshiki Sugimoto, Associate Professor at the Institute for Molecular Science, succeeded in directly and quantitatively demonstrating that the proton activity is significantly enhanced at the surfaces of low-temperature ice. On the basis of simultaneous experimental observation of the H/D isotopic exchange of water molecules at the surface and in the interior of double-layer crystalline-ice films composed of H2O and D2O, they reported three major discoveries of the unique enhancement of surface proton activity: (1) proton activity proved by the H/D exchange at the topmost surface is at least three orders of magnitude higher than in the interior even below 160 K; (2) the enhanced proton activity is dominated by autoionization process of water molecules rather than proton transfer process at ice surface; (3) as a consequence of surface promoted autoionization, the concentration of surface hydrated protons is estimated to be more than six orders of magnitude higher than that in the bulk.
Correlating these results with molecular-level structure and dynamics of the low-temperature ice surface, they discussed that the cooperative structural fluctuations allowed in the undercoordinated surface molecules but inhibited in the fully coordinated interior molecules facilitate the autoionization and dominate the proton activity at the ice surface. Because the lower limit of temperature of the earth's atmosphere is ~120 K around the mesopause, the surface of crystalline ice on earth is unlikely to be solidly ordered but would inevitably be highly fluctuated. In nature, such dynamic features facilitate the autoionization of water molecules and thus enhance the proton activity at the surface of crystalline ice. "Our results not only advance the physical chemistry of interfacial hydrogen bonds but also provide a firm basis for elucidating the key properties of ice surface that are of great interest in a variety of phenomena relevant to the dynamics of hydrated protons," says Sugimoto.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Early detection makes batteries safer - TU Darmstadt and MIT develop methods for monitoring with machine learning

The global run to mass LIB production: How the industry positions itself for cheap, sustainable and highly performant batteries - Europe on the way to self-sufficiency?
Lactobionic_acid
Allan_Nunn_May
Category:Arsenic_minerals
Category:British_biochemists

Trajan Group acquires Axel Semrau - The two Managing Directors at Axel Semrau and all other employees will continue in their current roles as part of the Trajan group
Neurotrophin
Category:Rubber_properties
1,1,1-Trichloroethane