BASF invests in its formulation site for food ingredients in Ballerup in Denmark
BASF has invested over € 6 million in the optimization and upgrade of the Ballerup site for food ingredient formulations in Denmark. Situated near Copenhagen, BASF’s Nutrition Ingredients business unit, affiliated to the Care chemicals division, produces high-quality, microencapsulated carotenoid and vitamin powders, supplying customers from the food, beverage, pharmacological and infant nutrition industries with the carotenoids beta-carotene, lycopene and lutein, vitamins A, D3, E, K1 and B12 and omega-3 fatty acids. The fortification of staple foods for developing countries, particularly vitamin A, has recently become another important business area.
"The investment allows us to respond even more flexibly and reliably to customer demand," comments Preben Sørensen, BASF’s site manager at Ballerup. "We now have cutting-edge process control technology, an additional packaging line and improved logistics. Furthermore, completely separate production lines ensure that products containing gelatin and those without gelatin don't come into contact, thus preventing possible contamination. That's an important concern for customers who supply vegetarian, allergen-free, kosher or halal products."
“Approximately 100 employees are now producing optimized, higher quality products even more efficiently and reliably at the Ballerup site, further increasing its market leadership in nutrition," says Dr. Martin Jager, head of BASF's Nutrition Ingredients business unit.
BASF's Ballerup site is certified in accordance with the Hazard Analysis and Critical Control Point (HACCP) system, which guarantees that the food ingredients manufactured there are safe for consumers. In addition, the site also operates according to GMP (Good Manufacturing Practice) guidelines, designed to assure the quality of production sequences. Ballerup was recently inspected by the competent food safety authorities and subsequently approved as a food production site. Furthermore, the so-called MUI halal certification, awarded by the Indonesian Ulema Council, and the kosher certification attest to BASF's compliance with Muslim and Jewish food laws in its production processes.
Topics
Organizations
Other news from the department manufacturing

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
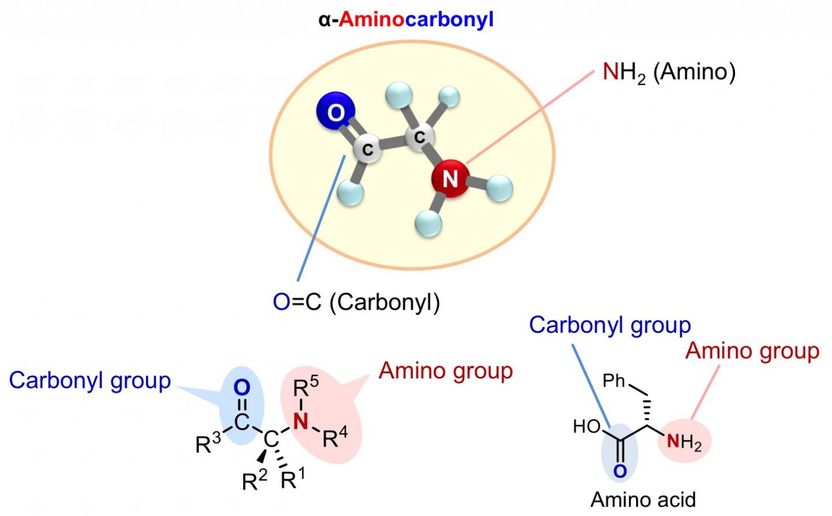
Rapid synthesis towards optically active alpha-aminocarbonyl therapeutics - New catalytic asymmetric reaction directly installs amines into carbonyl compounds
Zomepirac