New synthesis approach for soluble silicon clusters
Anticipating silicon solar cells with significantly improved efficiency
Theoretical calculations indicate that under certain conditions silicon can endow solar cells with a much higher efficiency. Small silicon clusters may provide a source of accordingly modified silicon. However, to date these clusters have not been accessible in soluble form, a prerequisite for flexible processing. Researchers at the Technical University of Munich (TUM) have now discovered a simple synthesis approach.

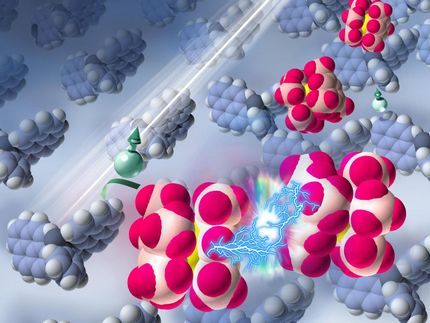
In a simple, two-step reaction scientists at the laboratory of Prof. Fässler produce nine-atom silicon clusters in soluble form.
U. Benz / TUM
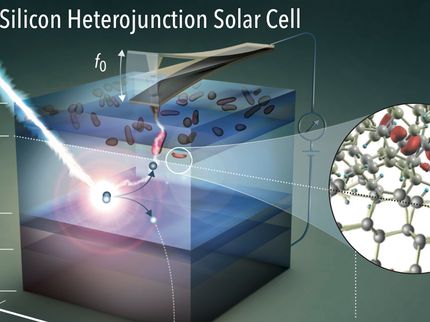
From nine-atom silicon clusters, larger structures should be achievable. Theoreticians hope to obtain materials that have a direct band gap and thus enable significantly more efficient solar cells.
A. J. Karttunen / Aalto Universität

Today, the best silicon solar cells in the world have an efficiency of 24 percent. The theoretical limit is around 29 percent. "This is because silicon normally crystallizes in a diamond structure which provides only an indirect band gap," explains Thomas Fässler, Professor of Inorganic Chemistry with Focus on New Materials at the Technical University of Munich.
Researchers thus dream of materials in which silicon atoms are arranged in a manner that creates a direct band gap that they can exploit for solar energy production. The scientists view these small silicon clusters as model compounds for this purpose because the atoms can be arranged differently than in crystalline silicon.
"These kinds of compounds are also interesting for a variety of other chemical experiments," says Professor Fässler. “Using only a few synthesis steps we can now join four and nine silicon atoms into tetrahedrons or near-spherical structures. However, in the past, the synthesis and isolation of the atomic clusters was very laborious. Now, we have taken a significant step forward."
A cluster of nine silicon atoms
Fusing potassium and silicon results in a compound with 12 potassium and 17 silicon atoms, a gray powder. First author Lorenz Schiegerl managed to stabilize the soluble, nine-atom clusters in liquid ammonia using a clever trick: He added an organic molecule to the ammonia that encapsulates the potassium atoms.
"This simple synthesis, starting from elemental silicon, opens the door to myriad chemical experiments with these clusters," says Professor Fässler. "In the solvent pyridine, for example, the cluster is stabilized by two hydrogen atoms, similar to the presumed intermediates in large-scale production of polycrystalline silicon, which is prepared using silanes or chlorosilanes for commercially available solar cell modules."
Creating new structures
Particularly promising is another reaction path to obtaining compounds made of silicon clusters. Here, three of the nine silicon atoms combine with molecules that in turn contain silicon or, for example, carbon or tin. Clusters with the highest currently known silicon concentrations are found in these reddish-brown solutions. This opens up novel possibilities for depositing silicon with modified structures out of solution.
"Extending this line of thinking, it should also be possible to build larger silicon structures using clusters of clusters. That would nearly close the gap to the aspirations of theoreticians," says Prof. Fässler. "In any case, we have opened the door to fascinating, new chemistry."
Original publication
L. J. Schiegerl, A. J. Karttunen, W. Klein, T. F. Fässler; "Silicon Clusters with Six and Seven Unsubstituted Vertices via a Two-step Reaction from Elemental Silicon"; Chemical Science; 2019.
L. J. Schiegerl, A. J. Karttunen, J. Tillmann, S. Geier, G. Raudaschl-Sieber, M. Waibel, T. F. Fässler; "Charged Si9 Clusters in Neat Solids and the Detection of [H2Si9]2- in Solution – A Combined NMR, Raman, Mass Spectrometric, and Quantum Chemical Investigation"; Angew. Chem. Int. Ed.; 2018.
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.