Researchers discover stable high-energy material
Advertisement
High-energy materials that can store very large amounts of chemical energy and release it when required are being sought for long-haul space travel worldwide. nitrogen compounds in which several nitrogen atoms are linked by single bonds possess this ability. These compounds are difficult to synthesize because they are highly unstable. Scientists at the University of Bayreuth have discovered a novel type of high-energy nitrogen specie under extremely high pressures and temperatures that remain stable under normal room conditions.
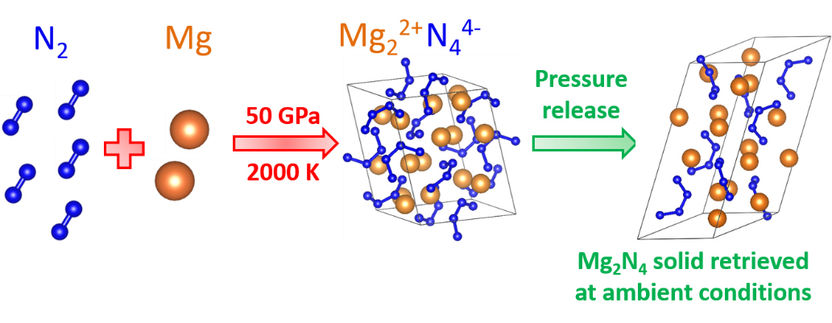
Synthesis of the new high-energy material.
Dominique Laniel
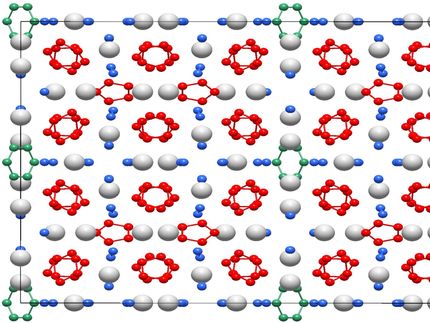
The Bayreuth team, led by Dr. Dominique Laniel, has stored a mixture of metallic magnesium (Mg) and nitrogen (N₂) in a diamond anvil cell. They then subjected the mixture to a temperature of 2,000 Kelvin (more than 1,700 degrees Celsius) and a pressure of 50 Gigapascal, pressure corresponding to 500,000 times the pressure of the Earth's atmosphere. Under the extreme pressure and temperature conditions, very unusual crystals of magnesium and nitrogen were formed, as was shown in experiments at the PETRA III X-ray source of the German Electron Synchrotron (DESY) in Hamburg. Among other things, the researchers discovered crystals with the chemical formula Mg₂N₄, which are composed of magnesium cations (Mg²⁺) and nitrogen anions (N₄⁴⁻). These homoatomic nitrogen molecules are composed of four single-bonded nitrogen atoms that form a horseshoe-shaped structure. This high-energy polynitrogen entity has never been synthesized before; whether through high pressure methods or conventional chemical approaches. Moreover, the scientist also observed the compound to survive the pressure release and be stable at ambient conditions.
"We were surprised when we noticed that these nitrogen ions embedded in the crystal structures remain stable at normal air pressure and room temperatures. The N₄⁴⁻ entity is only the fourth discovered polynitrogen unit and is the only one, so far, that can solely be obtained with high pressure technologies," said Dr. Dominique Laniel. The Bayreuth researchers are confident that a process for the synthesis of a polynitrogen-only compound can be developed. The result would be a high-energy material that is highly attractive for a variety of industrial applications and, above all, as an energy source for extended space travel. "Anyone looking for fuel to fly to Mars should look to polynitrogen compounds in the future," says Prof. Dr. Natalia Dubrovinskaia from the Bayreuth Laboratory for Crystallography.
However, a decisive hurdle still has to be overcome for these applications: To date, the magnesium-nitrogen crystals containing the high-energy nitrogen anions have only been able to be produced in very small quantities under extreme pressures and temperatures in the laboratory. A process for industrial-scale synthesis has not yet been developed. "It is quite possible, however, that the stable crystals produced in our high-pressure experiments will be suitable as blueprints to be reproduced one day using other, technically less sophisticated methods. In this respect, experimental high-pressure research is doing pioneering work in the search for high-energy materials," says Laniel. "With the research results now published in Nature Communications, the door is wide open to using high-pressure research methods to produce new high-energy materials of which we are currently unsure whether they can even exist," adds Prof. Dr. Leonid Dubrovinsky of BGI.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.