Overcome the bottleneck of solid electrolytes for Li batteries
Chinese scientists developed an atom-to-atom strategy
MA Cheng from the University of Science and Technology of China (USTC) and his collaborators proposed an effective strategy to address the electrode-electrolyte contact issue that is limiting the development of next-generation solid-state Li batteries. The solid-solid composite electrode created this way exhibited exceptional capacities and rate performances.
Provided by MA's team
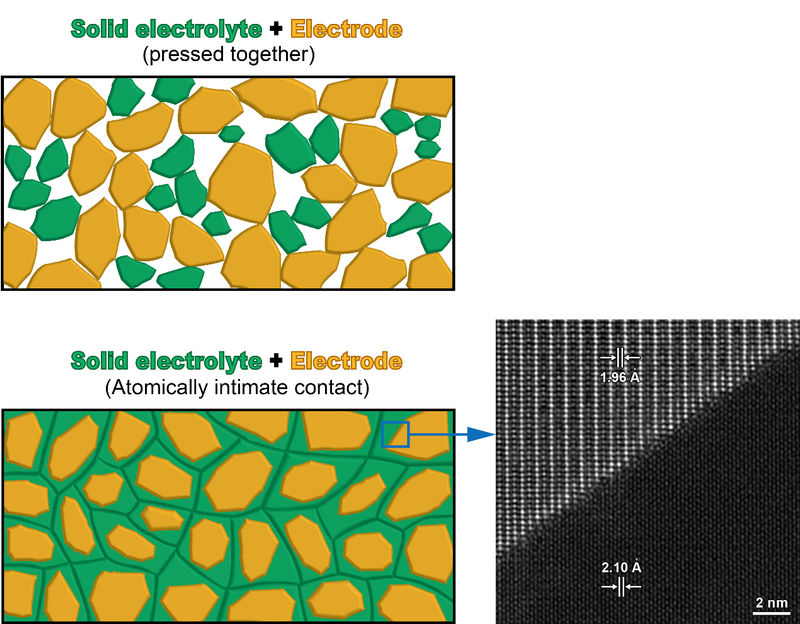
Replacing the organic liquid electrolyte in conventional Li-ion batteries with solid electrolytes can greatly alleviate the safety issues, and potentially break the "glass ceiling" for energy density improvement. However, mainstream electrode materials are also solids. Since the contact between two solids is nearly impossible to be as intimate as that between solid and liquid, at present the batteries based on solid electrolytes typically exhibit poor electrode-electrolyte contact and unsatisfactory full-cell performances.
"The electrode-electrolyte contact issue of solid-state batteries is somewhat like the shortest stave of a wooden barrel," said Prof. MA Cheng from USTC, the lead author of the study. "Actually, over these years researchers have already developed many excellent electrodes and solid electrolytes, but the poor contact between them is still limiting the efficiency of Li-ion transport."
Fortunately, MA's strategy may overcome this formidable challenge. The study began with the atom-by-atom examination of an impurity phase in a prototype, perovskite-structured solid electrolyte. Although the crystal structure differed greatly between the impurity and the solid electrolyte, they were observed to form epitaxial interfaces. After a series of detailed structural and chemical analyses, researchers discovered that the impurity phase is isostructural with the high-capacity Li-rich layered electrodes. That is to say, a prototype solid electrolyte can crystallize on the "template" formed by the atomic framework of a high-performance electrode, resulting in atomically intimate interfaces.
"This is truly a surprise," said the first author LI Fuzhen, who is currently a graduate student of USTC. "The presence of impurities in the material is actually a very common phenomenon, so common that most of the time they will be ignored. However, after taking a close look at them, we discovered this unexpected epitaxial behavior, and it directly inspired our strategy for improving the solid-solid contact."
Taking advantage of the observed phenomenon, the researchers intentionally crystallized the amorphous powder with the same composition as the perovskite-structured solid electrolyte on the surface of a Li-rich layered compound, and successfully realized a thorough, seamless contact between these two solid materials in a composite electrode. With the electrode-electrolyte contact issue addressed, such a solid-solid composite electrode delivered a rate capability even comparable to that from a solid-liquid composite electrode. More importantly, the researchers also found this type of epitaxial solid-solid contact may tolerate large lattice mismatches, and thus the strategy they proposed could also be applicable to many other perovskite solid electrolytes and layered electrodes.
"This work pointed out a direction that is worth pursuing," MA said. "Applying the principle raised here to other important materials could lead to even better cell performances and more interesting science. We are looking forward to it."
The researchers intend to continue their exploration in this direction, and apply the proposed strategy to other high-capacity, high-potential cathodes.
Original publication
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.