Better chemistry through tiny antennae
Advertisement
A research team at The University of Tokyo has introduced a powerful method for actively breaking chemical bonds using excitations in tiny antennae created by infrared lasers. This process may have applications throughout chemistry as a way to direct chemical reactions in desired directions. In particular, the reactions used in the energy, pharmaceutical, and manufacturing sectors may become much more efficient by increasing yields while reducing waste.
Institute of Industrial Science, the University of Tokyo
Chemistry is a messy undertaking, since there may be a variety of ways the starting chemicals can react, and each pathway might lead to the formation of a different product. Over the years, chemists have developed many tools--including changing the temperature, concentration, pH, or solvent--to nudge the reaction to maximize the yield of the desired molecules.
However, if given the ability to selectively control the making or breaking of individual bonds within a molecule, scientists could greatly enhance the efficiency of these reactions, while minimizing unwanted side products. "Being able to control chemical reactions on a molecular level--that is, the ability to selectively break or form chemical bonds, is a major goal for physical chemists," says first author Ikki Morichika.
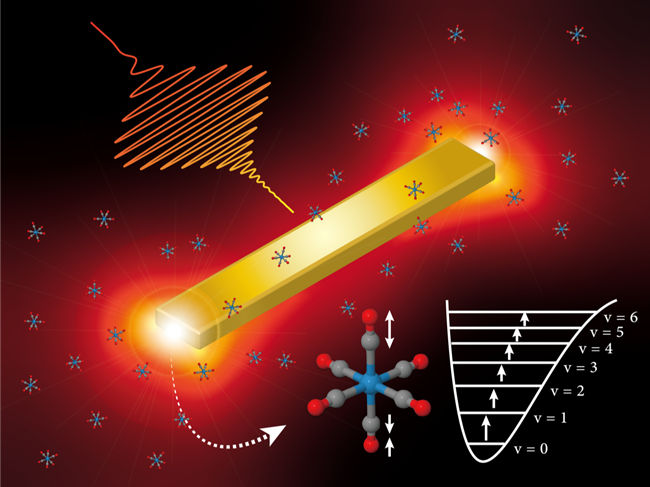
One way to control which bonds are broken during a chemical reaction is to get molecules vibrating by exciting them with infrared laser light. Since each type of chemical bond absorbs a particular wavelength of light, they can be activated individually. Unfortunately, it is difficult to deliver enough energy throughout the sample to generate the vibration intensity required. The team at The University of Tokyo was able to overcome this problem by fabricating tiny gold antennae, each just 300 nanometers wide, and by illuminating them with infrared lasers. When infrared light of the right frequency was present, the electrons in the antennae oscillated back and forth in resonance with the light waves, which created a very intense electric field. This phenomenon is called a "plasmonic resonance," and requires that the antennae be just the right shape and size. The plasmonic resonance focused the laser's energy on nearby molecules, which started vibrating. The vibration was further boosted by shaping the waveform of the infrared laser so that the frequency changed rapidly in time, reminiscent of the chirping of birds. "This successfully demonstrated that the combination of ultrafast optics and nano-plasmonics is useful for efficient, selective vibrational excitation," says senior author Satoshi Ashihara.
In the future, this technique may be applied to the production of cleaner fuels or cheaper pharmaceuticals as the chemical processes become optimized.